


3.3 Evaluation of Macroevolution (continued)
3.3.2 Rational Incoherency
a) Insufficiency of Natural Selection to Account for Macroevolution.
Evolution
above the species level has not been satisfactorily accounted for by the
mechanism of natural selection even though it does explain nicely the phenomena
of microevolution. This quote is from a recent text on evolution (18):
The process of natural selection, acting upon
the sources of genetic variability that reside in the gene pools of species,
is clearly adequate to produce, preserve, and accumulate the sorts of changes
that lead one species to another. There is a voluminous body of theory
and evidence to explain the origin of species through microevolution.
The differences between distantly allied species are
profound, however .... The differences between such species are in fact
so impressive that some investigators have suggested that they have arisen
through mechanisms distinct from the microevolutionary processes of adaptation.
This has led to some of the major controversies in evolutionary theory.
Various concepts have been proposed to account for macroevolution. The
following sections will review the concepts of neutral mutation, regulatory
mutation, systematic mutation, and species selection.
(1) Concept of Neutral Mutation. As mentioned earlier in 1.1.4,
the classical mutation theory was popular in the earlier part of the twentieth
century until the elaborate work of Dobzhansky and others who presented
a strong case for evolution by natural selection. The two theories predict
different degrees of genetic variability in natural populations. [216]
The classical theory predicts that most individuals in the natural population
are homozygous since natural selection serves to eliminate all but a few
mutations that are in the form of heterozygotes. Therefore, genetic variability
in natural populations, according to the classicists, is minimal.
The Neo-Darwinist, or the selectionist, on the other hand, predicts
exactly the opposite results. Since natural selection works on gene mutations
to cause changes in gene frequencies, many individuals in natural populations,
which are presumably undergoing evolutionary change, would be heterozygotes
that arise from mutations but are selected by overdominance (see
I.3.2.1). According to selectionists then, genetic variability in
natural populations is the rule instead of the exception.
Before the evidence from the studies of molecular evolution was available,
only mutations that had drastic phenotypic effects were analyzed, and the
phenomenon of genetic variability in natural populations could not be empirically
detected. Therefore, although examples of polymorphism (i.e., situations
where the members of a natural population can be sharply categorized into
two or more relatively common phenotypes that are determined by commonly
occurring alleles at a particular gene locus, such as blood types) were
recognized in genetics early in the science, it was impossible to tell
whether these represented special cases or a widespread phenomenon.
In the late 1960s, workers began to employ the technology of molecular
biology to tackle the problem of genetic variability in natural populations.
By the application of electrophoretic techniques, it was found that enzyme
and protein polymorphism both in Drosophila (19,
20)
and the human (21) is a common phenomenon. Similar studies
have been carried out in plants (22,
23)
and in animals, including protozoans, mollusks, arthropods, bryozoans,
echinoderms, and vertebrates (24). It has become clear
that this phenomenon is ubiquitous at least in the natural populations
of animals. The discovery of the widespread polymorphisms together with
the apparent constant rate of molecular evolution (see
I.3.3.1.d)
gave the classicists new impetus, and they postulated a new concept of
neutral
mutation in reformulating their theory. Presently, they are called
the neo-classicists
or
panneutralists
as contrasted with
the Neo-Darwinian school of selectionists.
There are two parameters in the description of enzyme or protein polymorphisms
in natural populations: (1) percentage of loci (alleles) that are polymorphic,
and (2) percentage of loci (alleles) that are heterozygous. Each parameter
can be accurately estimated by analyzing the gel electrophoretic pattern
(Figure 3.19) showing a typical analysis of enzyme [217] polymorphism.
The electrophoretic pattern can allow the differentiation of polymorphic
alleles and can show if an organism is homozygous or heterozygous at a
genetic locus.
The phenomenon of polymorphisms and heterzygosity is illustrated in
a study of enzymatic differences within a single species. Esterase-5
is
an enzyme known to be synthesized by the gene locus 5 in a particular chromosome
of Drosophila pseudoobscura. Sample 1 is a homozygous standard strain,
as indicated by a discrete protein band synthesized by two identical alleles
on locus est-51.00. The superscript stands for the relative
electrophoretic mobility of the protein specified by the allele. Samples
2, 3, and 6 have a slower migrating protein, while sample 5 moves faster
than the standard. Sample 4 shows three bands, two identical with the fast-
and slow-moving bands and one intermediate between [218] them. Samples
7-12 contain other proteins that are irrelevant to the present discussion.
By looking only at the mobilites and the discreteness of the protein bands
in sample 5 and samples 2, 3, and 6, one can infer that the two homozygotes
est-51.12
and est-50.95 are represented by the fast- and slow-moving
bands respectively. Thus, we can conclude that the esterase-5 locus
is polymorphic, because different homozygotes can be detected according
to their electrophoretic mobilities from the natural population of D.
pseudoobscura.
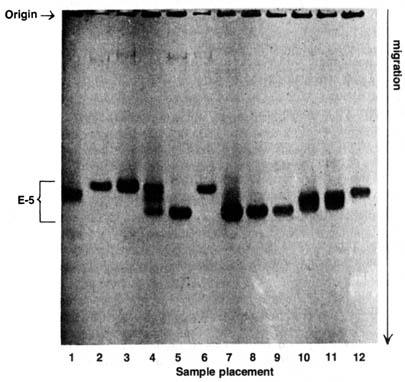
Figure 3.19. Adult esterases from Drosophila pseudoobscura.
Sample 1, standard strain est-51.00/est-51.00;
samples 2 and 3, est-50.95/est-50.95;
sample 4, est-50.95/est-51.12; sample
5, est-51.12/est-51.12; sample 6, est-50.95/est-50.95.
Reprinted, with permission, from Lewontin, R. C. The genetic basis of
evolutionary change. New York: Columbia University Press; 1974.
The three bands shown in sample 4 can be identified as a heterozygote
est-50.95
/ est-51.12 . The presence of the intermediate band in
sample 4 indicates that esterase-5 is a dimeric enzyme (consisting
of two polypeptides). Therefore, the heterozygote, est-50.95
/ est-51.12 can produce three dimers: (1) homodimer 0.95-0.95,
with the same mobility as the dimer made by the homozygote est-5^0.95;
(2) homodimer 1.12-1.12, with the same mobility as the dimer made by the
homozygote est-51.12 / est-51.12; and
(3) a hybrid dimer 1.12-0.95, with a mobility halfway between. This heterozygosity
of sample 4 is confirmed by the genetic analysis of individuals from the
strain with extracted protein of sample 4 that segregates into two different
alleles. Thus the electrophoretic pattern easily reveals the heterozygosity
of a locus regardless of the dominance or recessiveness of the alleles
because the product of each allele is examined.
Even when an enzyme is monomeric (consisting of only one polypeptide),
the heterozygote is easily detected also for it makes two different forms
of the enzyme. Each form corresponds to one of the homozygotes in mobility
despite the absence of the "hybrid" molecule as seen in sample 4. The different
enzyme forms produced by different alleles at the same locus are called
allozymes.
The difference of mobility in the various protein bands represents only
one-third or one-half of the amino acid changes in the polypeptides that
are responsible for the polymorphism. Many amino acid substitutions involve
no change in net charge of the protein and are not detected by the change
in electrophoretic mobilities. Therefore estimates of polymorphisms and
heterozygosities are only for the "lower limits" of what the case actually
is. From these data it is estimated that in sexually reproducing species
of animals, one-third of their genes are polymorphic, and 10% of the loci
of the individuals within the species are heterozygous (25).
The principles set forth by the neo-classicists to account for molecular
evolution are fivefold (26): (1) For each protein, the rate of evolution
in terms of amino acid substitutions is approximately constant per site
per year, as long as the function and tertiary structure of the protein
[219] molecules remain essentially unaltered. (2) Functionally less important
molecules or parts of molecules evolve (in terms of mutant substitutions)
faster than more important ones. (3) Those mutant substitutions that cause
less disruption in the existing structure and function of a molecule (conservative
substitutions) occur more frequently in evolution than do more disruptive
ones. (4) Gene duplication must always precede the emergence of a gene
having a new function. (5) Selective elimination of definitely harmful
mutants and random fixation of selectively neutral or very slightly harmful
mutants occur far more frequently in evolution than the positive Darwinian
selection of definitely advantageous mutants.
The first condition is somewhat substantiated by the development of
the molecular clock (see I.3.3.1.d, Figure 3.16) and the apparent
constant rate of evolutions in some proteins (see Figure 3.14).
Despite some disparities in the rate of evolution in some proteins, the
rate of neutral mutations is largely unknown because of limited data. However,
it can be calculated that the rate of gene substitution for neutral genes
is equal to the mutation rate, irrespective of population size (27,13).
Therefore, if mutation rate remains constant, the rate of evolutionary
change for a given protein would also occur with constant probability.
The second condition seems to be well documented by the fact that fibrinopeptide
and proinsulinpolypeptide C, both relatively useless proteins, evolve 18
and 11 times respectively, faster than cytochrome c, which is an
essential protein in the energy transport mechanisms (28). The evolution
of hemoglobin 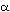 and
and 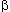 chain lends support to the third condition. The surface part of the molecules
evolves nearly 10 times as fast as the functionally important heme pocket.
chain lends support to the third condition. The surface part of the molecules
evolves nearly 10 times as fast as the functionally important heme pocket.
The fourth condition of gene duplication is a mechanism proposed initially
to explain protein evolution of hemoglobins and myoglobins that share many
common amino acid sequences and similar functions. It seems to be the most
appealing mechanism to account for the evolutionary acquisition of a large
amount of DNA in higher organisms (Figure 3.20). However, the theory of
gene duplication has no empirical documentation. The lack of apparent selective
values of most of the polymorphisms observed in natural populations are
taken to be indicative of the failure of natural selection to maintain
genetic variability of these loci. Last, natural selection is taken by
panneutralists as the editor, rather than the composer, of the genetic
message as claimed by the selectionists.
The controversies between panneutralists and Neo-Darwinian selectionists
can be represented in their disagreements over the interpretation of two
phenomena—the apparent constant rate of protein evolution and the widespread
protein polymorphism. First, the panneutralists claim [220] that the apparent
constant rate of protein evolution (which may be subject to different interpretations)
is strong evidence to support the hypothesis that it is the result of neutral
mutation, the rate of which determines the rate of amino acid substitution
by random genetic drift. However, the selectionists have to make very specific
assumptions on mutation rates, selective advantages, and effective population
sizes in order to explain the apparent constancy of protein evolution.
On the one hand, selectionists question the constancy of protein evolution.
On the other hand, they stress that amino acids in a protein sequence in
a species have been selected in the course of evolution because they are
best adapted to meet the particular features of external environment. Selection
of an amino acid [221] sequence of a protein also depends on the activities
of the other genes present.

Figure 3.20. The minimal amount of DNA that has been observed
for various species in the types of organisms listed. Each point represents
the measured DNA content per cell for a haploid set of chromosomes. The
ordinate scale and the shape of the curve is arbitrary. Reprinted, with
permission, from Nei, M. Molecular population genetics and evolution,
New York: Elsevier & N. Holland; 1975.
In considering the second phenomenon, panneutralists maintain that the
widespread polymorphism in natural populations is the result of mutations
that are neither beneficial nor harmful and are fixed by random genetic
drift. Natural selection is mainly responsible for the elimination of harmful
mutations and only occasionally establishes rare advantageous mutations.
Selectionists argue that the heterozygotes are responsible for carrying
the mutations and are selectively more advantageous (hybrid vigor or overdominance)
than the homozygotes. Therefore, protein polymorphisms are the expressions
of a stable genetic equilibrium maintained by balancing selection (see
I.3.2.1.c).
Both theories seem to account for some of the observations of molecular
evolution, but both have serious shortcomings. The panneutralists have
to document their assertion of the constant rate of protein evolution more
vigorously in light of the disparities observed in some known cases. In
addition, the proportions of allozyme polymorphisms showing selective values
are considerable and cannot be easily ignored.
The balancing selection theory is plagued with the conflict between
"hybrid vigor" and "inbreeding depression." If a large percentage of natural
populations are heterozygous as maintained by balancing selection, a significant
percentage of homozygotes would be derived by the inevitable inbreeding
of heterozygotes, and the genetic variability of the populations would
be reduced accordingly. However, this was not observed. Moreover, heterozygotes
produced by bisexual organisms seem not to be necessary for the maintenance
of protein polymorphisms for self-fertilizing plants (22)
and bacteria (29). Parthenogenic species of animals
(24)
are also shown to be polymorphic in many loci.
These data place severe strains on the selection theory. The rarity
of observable hybrid vigor, except the well-studied sickle cell anemia
(see
1.2.6.2;
3.2.1.c), also does not aid the selectionist's position. At the present
time the controversy between the selectionists and panneutralists goes
on with no solution yet in sight. It is fair to conclude that the foundation
of macroevolution based on natural selection is seriously shaken by the
panneutralists.
(2) Concept of Regulatory Mutation. The concept that major anatomical
changes are the result of mutations affecting gene expression (regulatory
mutations) was proposed to account for the apparent inconsistency between
molecular and organismal evolution (30) (see I.3.2.1.d).
According to this hypothesis, small differences in the time of activation
or in the level of activity of a single regulatory gene could in principle
[222] influence considerably the systems controlling embryonic development.
The organismal differences between chimpanzee and humans would then probably
result chiefly from genetic changes in a few regulatory systems that are
hardly detectable due to the difficulties involved in the purification
and identification of regulatory proteins.
Regulatory mutations can occur in two ways. First, nucleotide substitution
can affect a regulator gene that affects production but not the amino acid
sequence of proteins in that operon (see I.2.6, Figure 2.52). Second,
chromosomal rearrangements by inversion, translocation, duplication, deletion,
or transposition may be responsible for the change in genetic expression
without damaging the amino acid sequence of the gene products; however,
the biochemical mechanisms behind these changes are obscure.
The regulatory mutation concept is purely speculative, for there is
no empirical evidence to support it. The operon model is well documented
in the bacterial system, but it has not been unequivocally identified in
the eucaryotic genome due to the following four reasons:
1. DNA in the eucaryotic chromosome is wrapped in chromosomal
proteins that may play important roles in the regulation of gene expression.
The presence of these protein makes the identification of genes involved
in the operon very difficult.
2. Genomes of higher organisms contain various classes of highly repeated
DNA with virtually unknown functions.
3. A large part of the highly repeated DNA is apparently nonfunctional
because it does not transcribe any RNA.
4. The nucleus of an eucaryotic cell contains heterogeneous nuclear
RNA with high complexity (10 times that of cytoplasmic mRNA) that later
becomes mRNA in the cytoplasm. Several other low molecular weight species
of RNA with unknown function are also found in the nuclei.
Realizing the complexity of the eucaryotic genome (31),
scientists have yet to work out the regulation of gene expression. Therefore,
the concept of regulatory mutation can be treated only as a speculation
that is difficult to test empirically. At the present time it is very much
doubted whether the effects of regulatory gene mutation observed in bacteria
(32)
can be applied to the eucaryotic system.
(3) Concept of Systemic Mutation. The late Richard B. Goldschmidt
(1878-1958), geneticist at the University of California, has expressed
frustration in trying to account for the macroevolutionary development
of many structures in higher organisms on the bases of the mechanisms of
microevolution alone. He believed that the Neo-Darwinian mechanism (the
accumulation of micromutations under the [223] influence of natural selection)
was largely restricted to subspecific differentiation within species and
that the decisive step in the formation of new species involves an entirely
different genetic process called systemic mutation (33,
34).
Goldschmidt's reasoning on systemic mutation is threefold. First, if
microevolution gives rise to new species according to different stages
of geographic isolation, it should be possible to observe an entire series
of geographically isolated subspecies with the terminal one representing
the beginning of a new species. Goldschmidt expected to find these series
of geographically isolated subspecies of closely related species blending
into one another, but he cited many examples in which this blending did
not occur. He treated a species as an interbreeding or potentially interbreeding
population, and he claimed that many controversial cases of speciation
depend in part on the purely morphological definitions of a species that
do not take genetic aspects into account. Therefore he believed that good
species are always separated from their nearest relatives by a bridgeless
gap.
Goldschmidt's second point is that natural selection acting via geographical
isolation is believed to cause the accumulation of enough genetic difference
in the isolated subspecies so that it eventually becomes a new species
and is distinct from the parent species. But Goldschmidt documented in
many instances that long isolation did not produce more than subspecific
variations. He cited a race of the gypsy moth Lymantria dispar that
has been isolated on the island of Hokkaido (North Japan) since the early
Tertiary period, yet in the intervening 60 million years only subspecific
differentiation has occurred. He also pointed out that seasonal varieties
within a race of the butterfly Papilio may be greater than the variation
between races of Papilio butterflies at any one time.
Goldschmidt's third point is that the rate of evolution directed by
natural selection is too slow and subtle to account for the existing varieties
of plants and animals. Neo-Darwinian theory demands that only very minor
mutants subject to very slight selection pressure are significant in evolution.
If one defines selection pressure as the loss of survival value, then in
a population with 1000 individuals of AA genotype and 999 individuals
of aa genotype who reproduce, the selective pressure against a
is 0.001. According to mathematics set up by J. B. S. Haldane (35),
if a selection pressure of this magnitude is operating "in favor" of a
new gene that arose by micromutation present in frequency of one in a million,
it will take almost 12,000 generations to increase the gene frequency to
2 in a million if the favored gene is dominant, and 322,000 generations
if the favored gene is recessive. Goldschmidt inferred also from the lack
of [224] genetic differentiation of natural populations above the species
level such as in the case of Lymantria, which was under prolonged
and complete isolation, that natural selection is ineffective in speciation.
Goldschmidt therefore advocated a wholesale chromosomal rearrangement
that he called "the systemic mutation" as the novel genetic process to
account for speciation. Such a drastic chromosomal rearrangement is supported
by observations of Drosophila chromosomes. While natural selection
usually eliminates such individuals who arise from systemic mutation, occasionally
it may allow them to propagate as "hopeful monsters" under special circumstances.
The concept of systemic mutation lacks sufficient empirical documentation.
The only observable example seems to be polyploidy in plant speciation,
and this cannot be generalized to represent all living organisms. However,
the body of highly pertinent evidence amassed by Goldschmidt to support
his contention that the mechanisms of microevolution fail to account for
macroevolution has prompted a reevaluation of the roles played by natural
selection in the process of evolution.
Systemic mutation has also found its resurgence in a new theory called
punctuated
equilibrium. This theory is advocated by Stephen Jay Gould of Harvard
University and Niles Eldredge of the American Museum of Natural History
of New York (36). The essence of this theory is that
during evolutionary periods individual species remain virtually unchanged.
Speciation occurs only as "punctuations" caused by abrupt events at which
a descendant species arises from the original stock. This view finds increasing
acceptance among paleontologists who are dissatisfied with the imperfection
of the fossil record, which lacks many transitional forms.
The abrupt appearance of many animal body plans ranked as phyla and
classes approximately 500 million years ago has received more attention
in light of newly discovered fossil beds. It has drawn renewed interest
in what is so called the “Cambrian explosion” since the 1980s. The “top
down” pattern in which higher levels of biological hierarchy appear first,
or Disparity precedes Diversity, contradicts the Darwinian
prediction that lower forms should emerge before higher ones.(43)
Thus punctuated equilibrium has become a more dominant biological
theory. However, it still lacks the experimental status of a testable mechanistic
explanation.
(4) Concept of Species Selection. Reacting to arguments of opponents
of macroevolution, modern evolutionists have tried to reiterate their conviction
that the process of natural selection is responsible for both microevolution
and macroevolution. Yet the failure of natural selection (acting on individuals
within a population) to account for the major features of macroevolution
as represented by the fossil record has led to the formulation of the concept
of species selection (37).
It states that the random process of speciation favors species that
speciate at high rates and survive for long periods; therefore, they tend
to leave many daughter species. The concept was based on several extrapolations
from the fossil record: (1) The time a new species appeared to its extinction
or its pseudoextinction by gradually evolving into another species is between
six to seven million years. (2) The duration time for mammalian species
are shorter than those of some marine vertebrates with the major orders
of mammals arising from their primitive ancestors within a span not exceeding
[225] 12 million years. (3) During an interval not greatly exceeding five
million years, a new aquatic-animal family Limnocardiidae
arose
and developed over 30 new genera representing five subfamilies with members
showing great morphological diversity. (4) The presence of "living fossils"
such as the linguloid, brachipods, monoplacophoran mollusks, rhynchocephalian
reptiles, mytilid and pinnid bivalve mollusks, sclerosponges, and the lungfishes
indicate little or no evolutionary changes over a period of hundreds of
millions of years after their origination. These phenomena are not adequately
explained by the gradualistic accumulation of micromutations selected by
geographic isolation (allopatric speciation), but instead they demand a
rapid evolutionary mechanism to account for an initial fast apparent evolution
rate followed by little if any change.
The concept of species selection bears a certain resemblance to the
concept of sympatric speciation (see I.1.5) in that a sudden rapid
mechanism is responsible for the formation of new species. It is analogous
also to the process of natural selection in that species are selected on
the basis of their ability to resist extinction and to form a new species.
In contrast, natural selection occurs if individuals within a population
exhibit genetic variabilities caused by mutation and recombination. These
individuals are selected according to their abilities to survive and according
to their rates of reproduction. While the concept of species selection
may
account for the apparent rapid evolution of certain lineages represented
in the fossil record, it has no empirical documentation such as that of
the mechanism of natural selection in microevolution. Once again the mechanism
of natural selection fails to account for the major features of macroevolution
in the concept of species selection.
Recently, with the discovery of the uniqueness of Archaebacteria in
rRNA sequence and by comparative studies with well-characterized molecular
systems, cell walls, lipid compositions and features of the transcriptional
and translational machineries, the three domains of life, namely Archaea,
Bacteria and Eukarya, has become the currently accepted paradigm in the
field of molecular taxonomy.The three domains arise from an ancestral cellular
community (progenotes) undergoing high rates of mutation and
lateral gene transfer. The three domains crystallize into separate
cellular communities in this situation, becoming distinctive and refractory
to lateral gene transfer. In this fashion the domains are monophyletic
and entirely independent. The implication of polyphyletic origins of the
three domains of life from a universal pool of progenotes seems
to demand a mechanism beyond the realm in which Darwinian natural selection
canoperate.(44, 45, 46,
47,
48)
All in all, the idea of Darwinian evolution is still venerated as the
most comprehensive theory in biology. However, the concept of natural selection,
by which the theory was given a scientific basis, is being gradually abandoned
by the more radical biologists as the major mechanism that can account
for the features of macroevolution. In addition, a recent conference on
macroevolution has epitomized the growing contention that macroevolution
occurs by a mechanism other than natural selection (36).
The Darwinian evolutionists are no longer the dominating voice in the scientific
debate on evolution. R. C. Lewontin has summed up his evaluation of natural
selection as follows (38):
During the last few years there has been a flowering
of interest in evolution by purely random processes in which natural selection
plays no role at all. If the empirical fact should be that most of the
genetic change in species formation is indeed of this "non-Darwinian" sort,
then where is the revolution that Darwin [226] made? The answer is that
the essential nature of Darwinian revolution was neither the introduction
of evolutionism as a worldview (since historically that is not the case)
nor the emphasis on natural selection as the main force in evolution (since
empirically that may not be the case), but rather the replacement of a
metaphysical view of variation among organisms by a materialistic view.
In other words the only contribution that Darwin's natural selection theory
made is in the form of an empirically testable mechanism that causes the
diversification of genetic variability as exemplified by the process of
microevolution. Macroevolution, then, is a very speculative theory that
is becoming gradually divorced from the well-documented concept of natural
selection (42).
b) Chance as the Teleological Explanation of Evolution. Aristotle
has categorized four levels of explanation of an event: material cause,
efficient cause, formal cause, and final cause. A sculpture
can be used as an example. The material explanation of it is the stone
or wood from which the sculpture is made, i.e., the sculpture is a piece
of stone or wood. The efficient cause is the force, the act of carving,
that forms the figure of the sculpture, i.e., the sculpture is a carved
stone or wood. The formal cause is the pattern after which the sculpture
is carved, i.e., a sculpture is a carved stone statue of man. The final
cause is the purpose of the existence of the sculpture, i.e., the sculpture
is a carved stone statue of Abraham Lincoln, commemorating his work as
president of the United States.
According to Webster's Third New International Dictionary, the
use of design, purpose, or utility as an explanation of any natural phenomenon
is known as teleology. Thus, a teleological explanation of an event
is equivalent to Aristotle's final cause. The English theologian William
Paley argued eloquently in his Natural Theology that the intriguing
features of nature evidence the design of the Creator. He cited the example
of the human eye. Paley points to the fitting together efficiently and
cooperatively of the lens, retina, and brain; enabling humans to have vision;
as conclusive evidence of the design of an all-wise Creator. Thus the functional
design
of
organisms and their features are taken as evidence of the existence of
the designer.
Darwin rejected the notion of a designer and argued that the directive
organization of living things is the result of a natural process—natural
selection. In other words, he stressed only the material and efficient
causes of the features of the organisms and tried to bring the origin and
adaptation of organisms into the realm of empirical science. Evolutionists
maintain the natural process can be accounted for by physiochemical [227]
parameters; therefore, there is no need to resort to the design of a Creator
or external agent. Nevertheless, they invoked natural selection as an agent
capable of providing a purpose for the existence of certain features of
organisms. Evolutionists have on the one hand regarded the teleological
explanation of natural phenomena as untestable scientifically and thus
untenable; but they have asserted, on the other hand, that chance, directed
by natural selection, is the ultimate explanation of the necessity of evolution
(39).
The results of natural selection as exemplified by the adaptive feature
of the hand of humans, the wings of birds, and other biological structures
or behaviors have been treated as the reasons why they exist at
all. To put it more precisely, according to the evolutionists, the reason
why there are streptomycin-resistant mutants in a population of E. coli
bacteria is that they can propagate in the presence of the drug, whereas
streptomycin sensitive bacteria cannot. Since streptomycin-resistant mutation
arose spontaneously, the selection by the drug simply facilitates the differential
multiplication of the mutant at the expense of the nonmutated bacteria.
Therefore, according to the evolutionists, the "music of the biosphere"
is composed of the unaided "noise of natural selection" feeding on "chance"
alone (39).
Chance mutations can be subjected to the following fates: (1) selection
by favorable natural environments thus providing the raw materials for
further evolution, and (2) fixation by random drift or elimination by adverse
conditions thus moving to oblivion. The assertion that the present biota
is "entirely" the result of successful evolution of chance events by the
process of natural selection is purely a posteriori, since natural selection
is also known to cause extinction. The same assertion can be made to describe
a hypothetical barren earth as the result of natural selection of unsuccessful
chance events. Therefore evolutionists, while stressing the material and
efficient causes of evolution in the mechanism of natural selection, have
yet to come up with a valid counterargument to explain why chance alone
can be in such marvelous harmony to produce the orderly array in the biosphere.
Could not chance equally cause disruption of the whole structure? Both
of these phenomena would be equally probable conditions implicit in the
use of the term "chance."
c) Empirical Unfalsifiability of the Theory of Organic Evolution.
After the triumphant Centennial Celebration of Darwinism in 1959, a mathematical
and philosophical debate continued into the 1960s regarding the logical
coherency of Darwin's concept of natural selection. The arguments focused
on the circular reasoning of Darwin's premise of the survival of the
fittest. Darwin did not provide any objective criteria to [228] identify
the fittest other than observing the survivor (40).
Evolutionists tried to get around the apparent tautology of their theory
by redefining natural selection to mean differential reproduction that
gives rise to changes in gene frequencies. In other words, a particular
genetic variant confers higher fitness in a particular situation.
Therefore it will leave more offspring in the course of time. The catch,
however, is still present; i.e., the conclusion is essentially part of
the premise, since the fitness is measured by the capacity to leave more
offspring. Grease summarized this change as follows: "What have we? One
more tautology: well, after all what survives survives . . . . When the
theory [of natural selection] is summed up in a formula for measuring differential
gene ratios, you have a theorem universally applicable because it is empty,
totally comprehensive, because it expresses simple identity" (41).
The theory of evolutionary change as a consequence of natural selection
promoting the adaptation of organisms to their environments is empirically
demonstrable in the laboratory and in nature (see I.3.2). However,
the difficulties involved in defining adaptation operationally and
the subtle relationship between adaptation and fitness cast doubts on the
integrity of the theory of natural selection as an all-inclusive theory
to account for the origin of life. The fact is that the evolution
of life from a single origin, an assertion adamantly maintained by most
evolutionists, is more an a priori assumption than an empirically
falsifiable theory.
The attempts to analyze the rate of molecular evolution by comparing
amino acid sequences of proteins and determining the adaptive values of
protein polymorphisms in order to illuminate the neutralist-selectionist
controversy will yield nothing more than circumstantial evidence that will
be subject to reinterpretation. No one can design any experiment or collect
any amount of data from nature to falsify the claim that organic evolution
has occurred. The legitimacy of extrapolating microevolutionary
observations to macroevolution is increasingly being questioned (27,
28,
33,
38,
42).
The empirical unfalsifiability of the theory of organic evolution has
removed it effectively from the realm of empirical science. It is apparent
that theories of origins go beyond the limitations of verifiable empirical
science and thus require philosophical assumptions and leaps of faith.
We will now examine an alternative to the Darwinian theory based on a Christian
theistic world view.



References 3.3
18. Dobzhansky, T.; Ayala, F. J.; Stebbins,
G. L.; Valentine, J. L.
Evolution. San Francisco: Freeman; 1977:
233.
19. Hubby, J. L.; Lewontin, R. C.
J.
Genet. 54:57; 1966.
20. Lewontin, R. C. Hubby, J. L.
J.
Genet. 54:59-61; 1966.
21. Harris, H.
Proc. R. Soc. Lon.
Ser. B. 164:298; 1966.
22. Marshall, D. R.; Allard, R. W.
J.
Genet. 66:393; 1970.
23. Marshall, D. R.; Allard, R. W.
Heredity.
25:373; 1970.
24. Powell, J. R. In:
Evolutionary biology.
Dobzhansky, T.; Hecht, M. K.; Steere, W. eds. Vol. 8. New York and London:
Plenum; 1975: 29-119.
25. Lewontin, R. C.
The genetic basis
of evolutionary change. 118.
26. Kimura, M.; Ohta, T.
Proc. Nat.
Acad. Sci. USA. 71:2848; 1974.
27. Nei, M.
Molecular population genetics
and evolution. Amsterdam: N. Holland; 1975.
28. King, J. L.; Jukes, T. H.
Science.
164:788; 1969. [230]
29. Stanier, R. Y.; Wachter, D.; Gasser,
C.; Wilson, A. C.
J. Bacteriol. 102:351; 1970.
30. King, M. C.; Wilson, A. C.
Science.
188:107; 1975.
31. Hood, L. E.; Wilson, J. H.; Wood, W.
B.
Molecular biology of eucaryotic cells. Vol. 1. Menlo Park, CA:
Benjamin; 1975.
32. Hacking, A. J.; Lin, E. C. C.
J.
Bacteriol. 180:832; 1977.
33. Goldschmidt, R. B.
The material
basis of evolution. New Haven, CT: Yale Univ. Press; 1940.
34. Goldschmidt, R. B.
Am. Sci.
40:84; 1952.
35. Haldane, J. B. S.
J. Genet.
55:511; 1917.
36. Lewin, R.
Science. 210:883;
1980.
37. Stanley, S. M.
Proc. Natl. Acad.
Sci. USA. 72:646; 1975.
38. Lewontin, R. C.
Genetic basis of
evolutionary change. 4.
39. Monod, J.
Chance and necessity.
New York: Knopf; 1971: 118.
40. Eden, M.
Mathematical challenges
to the neo-Darwinian interpretation of evolution. Moorhead, P. S.;
Kaplan, M. M. eds. Philadelphia: Wistar Inst. Press; 1967: 5.
41. Grene, M.
Understanding of nature.
Essays in the philosophy of biology. Boston: Reidel; 1974: 84.
42. Stebbins, G. L.; Ayala, F. J.
Science.
213:967; 1981.
43. Wells, J. Icons of Evolution. Washington DC:
Regnery; 2000, Chap. 3, p. 29-58.
44. Doolittle, W. F. 2000. The nature of the universal
ancestor and the evolution of the proteome. Curr. Opin. Struct. Biol. 10:
355-358.
45. Woese, C. R. 1998. The universal ancestor.
Proc. Natl. Acad. Sci. 95: 6854-6859.
46. Woese, C. R. 2000. Interpreting the universal
phylogenetic tree. Proc. Natl. Acad. Sci. 97: 8392-8396.
47. Pun, P.P, Schuldt, S, and B. T. Pun. 2002. The
Three Domains of Life: A Challenge to the Concept of the Universal Cellular
Ancestor? Origins and Design. (In Press).
48. Woese, C. 2002. On the evolution of cells. Proc.
Natl. Acad. Sci. USA, Vol. 99, Issue 13, 8742-8747.

