 |
 |
IBRI Research Report #51 (2001)
Copyright © 2001, 2003 by David C. Bossard.
All
rights reserved.
ABSTRACT
| The universe was designed to make a place for humans to live. In an earlier talk we saw that it took about 10 billion years after the creation of the universe to prepare the ingredients to form the sun and the earth, and that in this process there are many indications of careful design that anticipate human habitation. This talk picks up at that point and looks at the next 4 billion years, and how the earth was made ready for the arrival of complex life - plants and animals - about 600 million years ago. We will see how microbial life figured in this preparation, and discuss the problems that the fossil record of this early life pose to natural evolution |
DISCLAIMER
| I have tried to be accurate in the statement of
scientific fact,
but it is possible, perhaps likely, that some factual errors have crept
in. I would appreciate any corrections of factual errors. Please email
them to me at dcbossard@valley.net.
In particular, I solicit additional citations to work available over
the
internet, that may supplement or clarify some of the matters discussed.
-- DCB
|
EDITOR'S NOTE
| Although the author is in agreement with the doctrinal statement of IBRI, it does not follow that all of the viewpoints espoused in this paper represent official positions of IBRI. Since one of the purposes of the IBRI report series is to serve as a preprint forum, it is possible that the author has revised some aspects of this work since it was first written.† |
A Fit Place to Live:
Creation of the
Biosphere
For this is what the LORD says--he who created the heavens, he is God; he who fashioned and made the earth, he founded it; he did not create it to be empty [a chaos, NRSV]1, but formed it to be inhabited--he says: "I am the LORD, and there is no other."In this talk we will see how God prepared the Earth for human habitation, as revealed in the geological and fossil record. We will begin with the formation of the Earth and end at the point where plants and animals first appear, the topic of a future talk.Isaiah 45:18, NIV
As in earlier talks2, I accept the general time line of events that took place early in Earth's history, as it is understood by most scientists.3 We will talk a bit about why that time line is correct. It indicates that nearly 4 billion years passed on earth before plants and animals appeared.
I agree that it seems almost incredible that we can stand here and say - as we will today - that specific things happened billions of years ago, and say it with confidence. But I believe that this reflects God's deliberate design. He planned to have his creation open up to careful scientific investigation. He did not have to do this: if God's work had been designed just a little differently, much of this history would have been hidden from view. We will see a remarkable example of this when we look at the early fossil record. So I accept it as a fulfilment of God's claim in Psalm 19:1-4 that begins: "The heavens declare the glory of God and the skies proclaim the works of his hands."
As Isaiah states in the above quote, God formed the earth to be inhabited. No firm evidence exists that any other planets or satellites host life as complex as plants and animals, and in fact, they could not support such life without the same special preparation that we will discuss here.
I should note that I try to avoid distinctions such as primitive/advanced, simple/complex and lower/higher when referring to the various living species. The reason is, as we argue both here and in earlier talks, that all life is exceedingly complex. Many popular discussions including some mentioned in the bibliography, seem to me to gloss over this complexity (for example, discussions of the earliest life on earth or of the possibility of life on another planet). I will, however, use the accepted technical terms when necessary, even though they also at times convey false impressions - for example, the archaeobacteria are not necessarily ancient or primitive although the term implies this. When I refer to multi-celled, oxygen-breathing species that live on land or in water, I will use the term "plants and animals." In this talk, a "fit place" is a place where plants and animals, and humans in particular, can thrive.
1. What does a Fit Place Require?
What does a fit place for life require? Here are some things that we will keep track of in this talk.
So in your mind, project yourself back 4.5 billion years to the time that the earth was first formed. How would you go about preparing the earth so that it is congenial to life? That is what we want to discuss today.
We are entering into a battleground area between those who believe in the God revealed in the Bible, and those who believe that there is no such God, whom we may call secular scientists or secular evolutionists. Many, perhaps most, scientists who work in the area of evolutionary biology and the fossil evidence are of - or present themselves as - the secular sort.
And there is also another unfortunate battleground among those who accept the authority of the Biblical creation account (as I do), between the so-called young earth and old earth creationists. I will not say much about this, except to give some comments on dating methods and why they are valid. If you find that what I say here differs from what you have heard, I urge you to look into these things for yourself and form your conclusions as I have.
2. Remarks on Radioactive Dating.6
Before we begin the account of the first 4 billion years, I would like to say something about how scientists determine the age of the Earth and early fossils and rock formations. These early dates use radioactive dating.
Certain elements, particularly the heavy elements, are slightly unstable, and they decay into lighter elements at a predictable rate. The half-life is used to describe this decay. The half-life is the amount of time needed for half of the element to decay.
Elements with half-lives on the order of billions of years are
useful
to measure ages on the order of billions of years. Some of these are
listed
in the following table. Elements with shorter half-lives are useful to
measure less ancient ages. Carbon-14 dating, for example, is only
useful
for ages up to about 100,000 years.
|
|
|||
| Element | Daughter | Half-life (yrs) | Effective dating range |
| Uranium-238† | Lead-206† | 4.51 billion | 10 million to origin of Earth |
| Uranium-235 | Lead-207 | 0.704 billion | 10 million to origin of Earth |
| Rubidium-87 | Strontium-87 | 48.8 billion | 10 million to origin of Earth |
| Potassium-40† | Argon-40 | 1.25 billion | 100,000 to origin of Earth |
| Carbon-14 | Nitrogen-14 | 5730 ± 40 yrs | 0-100,000 years |
Radioactive dating indicates when rock such as lava solidified, provided that the rock has not been contaminated after solidifying.8 For example, zircon crystals that form as lava cools are useful for radioactive dating. Zircon crystals capture radioactive uranium but not lead as they form, and so any lead found in the crystals is due to uranium decay.
Let me just say flat out, that I believe the half-lives listed in this table are an example of God's gracious providence. They are examples of the meaning of Psalm 19:1-4, especially beamed at the modern age of science. God wants us to be able to probe how he created life, and so he arranged things so that a few elements would have decay half-lives that are in the billions of years. This is not the only use of these elements, of course - for one thing, the Earth's core has been heated by radioactive decay since its very beginning, and that is one reason why it was warm enough to support life from the first. But it strikes me as much more than accidental that these elements are so useful in determining the ages of ancient rocks.
3. The Early Earth: the Hadean age, 4,550 to 3,800 Million Years (My) Ago10.
The first age of the Earth is called the Hadean age (from the Greek word Hades = Hell), the period of time when the Earth first formed, became a hot molten mass, and then slowly cooled to a temperature below the boiling point of water.
a. Formation of the Solar System and Earth. The solar
system
formed about 4,550 million (4.55 billion) years ago from the remnants
of
a supernova explosion. As the Sun formed from the large amount of
helium
and hydrogen left over from the supernova, its gravity captured many
supernova
fragments. Planets quickly grew as they swept out the fragments in
their
path. The Earth was formed over a period of 10-20 My, while the larger
gas planets, Jupiter, Uranus and Saturn, took a bit longer.
 |
Origin of the Solar System
Painting by William K. Hartmann.
Used by Permission. 12
We can date these events with considerable accuracy. The oldest meteorites formed when the fragments of the supernova solidified, and show ages of 4,550±25 My11.
Heat caused by impacts and radioactive decay of heavy elements melted the early Earth. During the Hadean era, this heat was far greater than the heat provided by the Sun. Melting caused the heaviest elements to sink to the core and the lighter elements to form the mantle and a thin crust.
b. Formation of the Moon. Shortly after the Earth formed,
it collided with another planet about the size of Mars, which
apparently
had also formed at about the same distance from the Sun. This collision
was fairly late in the planet formation process, so both planets had a
molten core.
 |
Formation of the Moon
by collision of the Earth with a Mars-sized Object
Painting by William K. Hartmann.
Used by Permission. 12
 |
Formation of the Moon
Five hours after collision
Painting by William K. Hartmann
based on computer simulation.
Used by Permission. 12
The melted cores combined into the Earth's core and orbiting fragments of crust formed the Moon13 (which accounts for the fact that Moon rocks have mostly lighter elements similar to the Earth's crust). The oldest moon rocks are 4,500 to 4,520 My old14, so the Moon was formed some 30 My after the Solar system first formed. These moon rocks were gathered by Luna 20 (U.S.S.R., February, 1972) and Apollo 17 (U.S.A., December, 1972).
At first the Moon was very close to the earth, perhaps 15,000 miles away. This caused huge tides, a hundred times more violent than we experience today, in both the Earth and the Moon.15 The tidal friction stopped the Moon's rotation and caused it to recede from the Earth. Even today, the Moon recedes from the Earth about an inch a year, due to tidal friction.16
c. Age Records on the Moon. The Moon craters give a record of the chaotic times after the Moon formed. Some of the larger Moon craters may have formed as its receding orbit swept up some large fragments left over from the original collision.17 This bombardment ceased about 3,800 to 3,600 My ago18, at the end of the Hadean era.
The Moon's visual features give the timing of these impacts18a. The largest of these, the dark maria (seas), are huge lava flows from impacts and partial melting. They are also the most recent features, evident by the fact that they do not have the rich pattern of craters within craters that characterize the lighter-colored (and older) Moon highlands.
The following figure shows a series of Ranger 9 (U.S.A., March,
1965) photographs as it approached the Alphonsus crater located in the
highlands. The pattern of craters within craters, shows impacts over a
period of time. Part of Mare Nubium is shown in the figure, to the left
of Alphonsus.19
 |
The most recent of the large impact craters is Orientale, a
crater
formed about 3,800 My ago on the Moon's far side just beyond the
Western
margin of the visible near-side. The concentric rings are
characteristic
shock waves of the impact. The crater is about 600 km in diameter.
 |
Orientale Crater on the Moon's Far Side
(The Mare and two craters in the upper right are visible
from Earth at the Western Margin.)20
Several authors have remarked that the combination of the Earth and a massive nearby Moon are needed to make a place fit for life21. The tides mix up the oceans, distribute life and nutrients, eliminate biological dead zones, and contribute to the global weather patterns.
At the end of the Hadean period, the Earth had a crust, but it was thin and constantly being re-formed and broken up. The surface of the Earth at this time was too smooth (not enough highs and lows) for permanent continents (the Biblical dry land) to form - that will take billions of years to happen.
The sky was very dark and cloudy. Violent storms and volcanoes were a constant feature due to the Moon's close proximity that caused huge tidal forces to heave and wrench both the oceans and the solid crust. The seas were very salty (more so than the present oceans - later on, much of the salt precipitated out, as we will shortly see) and covered almost all of the Earth's surface. The only visible land was tips of volcanoes that poked above the water, but quickly eroded because no coral reefs or other stable structures protected them from severe tidal action. The atmosphere had almost no oxygen. It consisted primarily of nitrogen gas, with carbon dioxide, water vapor, ammonia and hydrogen sulfide in small amounts.
The Hadean period ended at about 3800 My, when the bombardment of debris and meteorites largely ceased. A fitness scorecard at this point shows some progress: the moon provides mixing, there is liquid water and some (but not much) available nitrogen produced by the constant lightning in the form of ammonia and some nitrogen-containing salts. There is very little oxygen. As the earth cools from a molten state, all available oxygen gas combines to form oxides or water, because hot matter very quickly uses up any available oxygen. That is what happens when you start a fire by applying heat: the fuel becomes hot enough that it spontaneously combines with atmospheric oxygen.22 The question then becomes, how can oxygen be put into the atmosphere and how can a reliable supply of nitrogen and other nutrients be introduced so that plants and animals can exist? This is done by bacteria.
4. The First Life appears: The Early Archaean Age, 3,800 - 3,400 My ago.
It was long assumed by biologists that no actual fossils of the earliest living species of life would be found. The reasons seem self-evident: the first life would be "simple", single-celled, and microscopic. It would be soft-bodied, with no skeletal parts, and very vulnerable to destruction by heat or decomposition. The shape would almost certainly be so basic - spherical or rod-like - that it would be virtually impossible to identify the remains as biological; they could just as easily be bubbles or minute stress-marks in the rock23.
Besides all of this, there is very little early rock exposed on the surface of the Earth. Most old rock has long since been pulverized by weather or buried by younger rocks. There are only a few places where old rock remains: small amounts in Greenland, South Africa and Western Australia.
So in the face of these expectations, it is utterly amazing that in the 1970s the earliest known fossils were found and identified by J. William Schopf in Western Australia, and dated to 3,465 My ago with an error of about 5 My. This is almost unbelievable accuracy for such an ancient date.
Schopf discovered the fossils at the famous Bitter Springs fossil site in Western Australia (known mostly for fossils younger by about 2 billion years).
How could the dating of these fossils be so accurate? The answer is a providential combination of events. These earliest fossils are sandwiched between two layers of lava that contain tiny zircon grains that could be dated very precisely. The lava layers were separated in time by about 12 My, so the fossils sandwiched between them could be dated to an acccuracy of about 5-6 My. This is marvelous and unexpected.24
Are these fossils primitive spheres or rods? Yes and no. They look like shriveled strings of rod-like cells as in the following sketch:
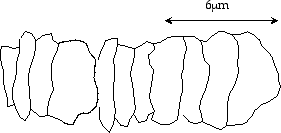
Typical fossil cyanobacteria25
They appear to be cyanobacteria, also called blue-green algae. A
typical modern example is Anabaena.
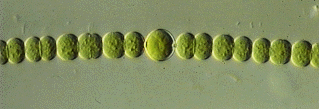 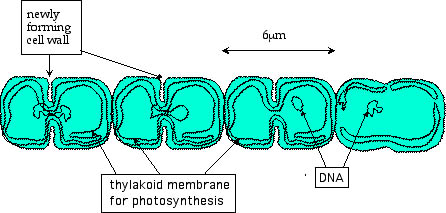 Anabaena, a Modern Cyanobacteria26 |
Cyanobacteria are moss-like species that live in oxygen-poor environments bathed in light, such as in shallow bodies of water. They are exceedingly complex, far from what one would think to call primitive. They grow in long chains because when the cells reproduce they divide in half and tend to remain attached. They secrete a kind of mucilage or slime which solidifies to form characteristic multi-layered dome-like structures called stromatolytes that grow in shallow water between high and low tide. Living stromatolytes exist today in only a few locations worldwide, one being Hamelin Pool in Western Australia.

Location of stromatolytes

|
Living Stromatolytes,
Hamelin Pool, Western Australia27
The earliest living species had to be complex because they had to survive on a very harsh and inhospitable earth. This need for complexity in the earliest living species is a basic dilemma for evolutionary theory.
Here are some of the problems facing the first living species:
a. They must be protected from lethal solar and cosmic rays.All of these problems had to be solved by the very first living species, which is why they must be very complex, and why undirected natural evolution is completely unable to account for the arrival of life on earth.
b. They must have a source of energy to carry on cellular activity
†- in just right amounts: not too much or too little.
c. They must have food
†- specifically sugar, to store energy and to make the carbon chains necessary
for life.
d. They must have nitrogen in a useful form, as ammonia, nitrates or proteins.
†- Nitrogen is absolutely basic to all of the genetic code and to the amino acids
that make proteins and the enzymes needed to make carbon chains.
e. They must be able to survive environmental extremes, such as freezing, famine and dehydration.
Here is how the earliest known fossil species solved each of these problems 3,465 My ago:
a. Protection from lethal rays. The early atmosphere did not filter out harmful rays. Cosmic rays are typically in the far ultraviolet spectrum. They are about five times more powerful than light photons, and can rip apart the life molecules. This is why they are so harmful to life.28
Fortunately, a few feet of water is a very good filter. So all of the early species of life lived in water - for the next 3+ billion years! By living in rigid stromatolytes, in shallow water, they could withstand the severe storm, wave and tidal action, enjoy sunlight, and take advantage of water movement to supply fresh food and remove waste material. Even the relatively large numbers of bacteria that may have been destroyed by harmful rays contributed food to the survivors.
b. Energy Source. Visible light is a good source of energy, but it is far too energetic to be useful in subtle cellular chemistry. The energy involved in an organic bonding is typically around 1-7 kcal/mole. This is a little higher than thermal energy (0.2 -0.7 kcal/mol at normal temperatures) but much less than light energy which is about 60 kcal/mol.29 For this reason, the energy in light is not directly useful because it is too powerful. It is like using a sledge-hammer to hit a tack.
So the first life that appears in the fossil record invented photosynthesis. This is a complex process that is built around a chlorophyll molecule. At the heart of chlorophyll is a magnesium atom suspended between 4 nitrogen atoms. This complex of atoms absorbs light rays and converts it into a flow of electrons and protons.
A useful picture of how chlorophyll works is to think of a pachinko or pinball machine. A plunger (the photon) hits a ball (an electron) and propels it into a high energy state. The magnesium/nitrogen complex is strong enough to capture this high energy electron in a high energy state.
The ball (electron) then falls back in a number of steps, in effect dividing the initial large energy into a series of smaller amounts of energy as it returns to its initial rest state. These smaller amounts of energy can then perform useful biological work.
The signifance of photosynthesis can hardly be over-emphasized. Without it, life simply could not exist on the early earth, for lack of a plentiful source of useful energy. It has been called the most important innovation on earth.30
Photosynthesis is an amazingly complex and intricate invention, involving more than 200 genes and a genetic code of over 150,000 base pairs.31 It involves two steps, called the light reaction and the dark reaction. The light reaction converts the photons to useable energy, and the dark reaction then uses that energy to make glucose sugar.
The light reaction decomposes water into oxygen, hydrogen ions (protons) and electrons:
2H2O -> 4e- + 4H+ + O2.
Note the products:
Photosynthesis uses the proton current to power a miniature rotary motor molecule called ATP synthase.
- Electrons: These are the basis of normal electric current.
- Hydrogen ions: These are protons32, and form another kind of current.
- Oxygen: This is a waste product.
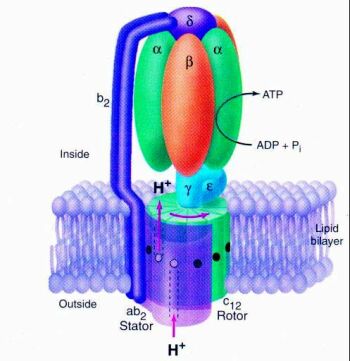 |
ATP Synthase "Motor"33
The motor, ATP Synthase, binds small packets of energy into a molecule called ATP (adenosine triphosphate). The energy packets are just the right size for the subtle chemistry found in living cells. A flow of protons passes through the motor and causes it to rotate. Under ideal circumstances, this motor can rotate at up to 18,000 rpm - about 6-10 times faster than a car engine - and produce 3 ATP molecules for each revolution.35
c. Food Production. The electron current in photosynthesis is used to make a molecule called NADPH, which in turn makes sugar. This is the dark reaction of photosynthesis. The process is called the Calvin cycle, named after M. Calvin who received a Nobel Prize in 1961 for its discovery.
d. Nitrogen Fixing. Nitrogen fixing is a very slow process. To convert a single molecule of nitrogen gas to ammonia, the nitrogenase molecule, which is made up of two giant proteins, must physically separate and rejoin eight times, and this takes about 1.2 seconds. Nitrogenase is also very scarce. All the world's supply of nitrogenase could be carried in a single bucket.34a Perhaps its not surprising that nitrogen-fixing bacteria had to work for billions of years to make enough nitrogen available for plants and animals to thrive.
But there is another problem here, besides the difficulty of fixing nitrogen, because the production of ATP in photosynthesis has oxygen as a waste product. Although cyanobacteria can do both photosynthesis and nitrogen fixing, these two processes cannot be done together because the oxygen poisons the nitrogen fixing process! So how can cyanobacteria both make ATP and fix nitrogen?
The solution is that some cyanobacteria specialize. They form a
thick cell wall that protects the interior from oxygen and then they
specialize
in nitrogen production, using ATP food produced by other cyanobacteria.
These specialized cells are called heterocysts; they are large cells
placed
every 10 cells or so along a chain of cyanobacteria. One author
describes
the formation of heterocysts in Anabaena (a species of cyanobacteria)
as
follows:
"Anabaena starts off as a filament of vegetative cells. When there is not enough fixed nitrogen to go around, one vegetative cells becomes a heterocyst. Heterocyst differentiation takes approximately 24 hours. But they are dead end cells: unlike their vegetative counterparts they can no longer multiply. However, the vegetative cells surrounding them continue to multiply. Once again when there is not enough fixed nitrogen to be found, another heterocyst appears. The result is a string of vegetative cells interrupted by heterocysts at quite regular intervals, roughly about every 10 to 15 vegetative cells."35a
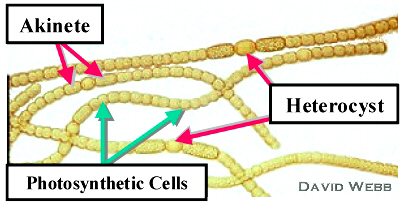 |
†Nitrogen-fixing Heterocysts
in a chain of cyanobacteria (Anabaena)
Used by Permission of David Webb.36
Within the colonies of cyanobacteria, the bacteria specialize and share their food: nitrogen, sugars and ATP, as well as protein contributed by dead bacteria. You can see why they must live in rigid colonies rather than as free-living bacteria, and you can see why they grow in chains.
e. Survival. There is one problem remaining: the early earth had severe and sometimes sudden changes in the environment. And for stromatolyte colonies, the shallow tidal zones that they require are constantly changing. So the first living species had to have a way to survive in a dormant state between times that they could flourish.
The solution is another kind of specialized cells called
akinetes,
which have thick walls and are able to survive freezing, starvation and
dehydration for long periods of time - perhaps even millions of years!
 |
Bacteria can even survive the cold and vacuum of space. A television camera left on the moon by Surveyor 3 (U.S.A., April, 1967) was retrieved on the Apollo 12 mission (U.S.A., November, 1969) over 2 1/2 years later and found to have live bacteria in a dormant state.39
This survivability of bacteria is perhaps why God chose to use bacteria to make the Earth a place fit for later, more complex species.
So the very first living species recorded in the fossil record at the crack of dawn already had solved some of the most complex problems of life:
We do not have time to mention other inventions, in particular the uses of membranes, which serve many important and diverse functions. The ATP synthase motor, for example, must be embedded in a membrane in order for it to work: a potential across the membrane is what causes the protons to flow through the motor.
In the process of doing all of this, the species produces oxygen as a waste product. The oxygen is of little direct use by these first living species - in fact it is a poison (oxygenation is the principal way that municipal waste water is purified of bacteria) - but without oxygen, higher life would never have been possible. This itself flies in the face of evolution, because the oxygen had no direct survival value to these bacteria. It's as if the earth was being prepared for something new and entirely different.
The fitness scorecard at the end of this period, 3,400 My ago, shows a little advance towards a fit place to live: the main change is that fixed nitrogen is being made by a biologically controlled process.
5. Preparing for more complex life 3,400 - 1,600 My ago
Bacteria are complex creatures, but they do not have what it takes to make plants and animals. For one thing, they can only be very small. Bacteria must be small because they rely on ordinary diffusion to move nutrients and wastes around in the cell. This limits the practical size of the cell. In addition, bacteria cannot form multi-celled species (we will discuss the reasons later).
On the other hand, plants and animals could not have existed on the earth at the time that bacteria were created. So bacteria were the first living species, and their main function for the next two billion years was to prepare the earth for plants and animals, a job that they continue today.
This is not the sort of goal that evolution would head towards, because, for one thing, there is no evolutionary reason for bacteria to build up the level of oxygen in the atmosphere. How could bacteria "know" that there is an advantage in building up reserves of a poisonous waste product?
Bacteria, because of their genetic make-up, are particularly well-suited for the harsh conditions of the early earth. They are able to adapt to varying circumstances (much better than the much more complex species that come later), to the point where one biologist, Lynn Margulis argues that the concept of species is not appropriate for bacteria. Different species of bacteria can even exchange genetic code. One author explains it this way:
†- Bacteria are genetically much less static than plants and animals - a constant buzz of genetic traffic within and between microbes. The traffic takes many routes. Phages, the viruses that infect bacteria, often inject their genes into a host's DNA, sometimes their own, and sometimes genes kidnapped from earlier hosts. Also bacteria can absorb floating fragments of DNA from their surroundings, even the genes of other bacterial species. For instance, the emergence of a new strain of E. coli results from this species picking up a toxin-producing plasmid from another type of bacteria.Note that this exchange of genetic code is possible because all living species use the same method for genetic coding, unlike the case with, for example, computer programming. The genetic flexibility that this quote talks about is essential so that the bacteria can adapt to changing environments, sometimes very severe.Arno Karlen, Biography of a Germ41
Three major tasks had to be done before more complex life was possible. These are:
a. Buildup of fixed nitrogen and other organic food.a. Food and Nitrogen The production of the nutrients from scratch is so slow that animals need ready-made organic food to keep going. The high level of biological activity in a typical animal cell cannot be met by making all of the cell's food from simple molecules. Humans, for example, require many organic nutrients - the vitamins are an example - that their own cells cannot make. This is not just laziness on the cell's part: it is because the cell already has so much to do, and because the production of the nutrients from scratch is slow. So one job of the early bacteria was to distribute ample nutrients worldwide in preparation for more complex life.
b. Buildup of a stable supply of atmospheric oxygen.
c. Creation of stable dry land.
b. Atmospheric Oxygen The early earth's crust was poor in oxygen for reasons that we have already mentioned. The earth's oxygen was tied up in water molecules or in partially oxidized solids.
For a long time after cyanobacteria started making oxygen as a waste product, it was removed quickly by oxidizing these oxygen-poor minerals. The oxygen supply went through many boom and bust cycles. It would build up, perhaps saturating the local environment, the bacteria would then die off, growth would stagnate, and the excess oxygen would gradually be converted to oxides. Then the cycle would repeat. The cycles lasted for nearly two billion years.
For the first billion years, uranium salts absorbed the oxygen. Then most of the available uranium was fully oxidized, and for the next billion years, silicon and iron soaked up the excess oxygen, forming sand and the great iron ore deposits.
The precipitation of silicon and iron is sensitive to the acidity of the environment. When acidity is high, silicates remain dissolved in the ocean water, but iron oxide precipitates. When acidity drops, the silicates precipitate out. These boom and bust cycles can be seen in a geological record known as the banded iron formations.

Banded Iron Formation of
iron oxide (Fe2O3= dark),
and silica (SiO2 = light)42
The banded iron formations end when most of the exposed crust was oxidized, and then the oxygen rose to a fairly stable 20-25% level in the atmosphere, where it has stayed ever since.
You may immediately see a potential problem: oxygen is generally poisonous to the bacteria that are the only life on earth. As long as there were minerals to draw off the excess oxygen, things could go on. But now, the earth's crust is fully oxidized. What is going to keep the oxygen from growing to the point where life hits a stagnant and unfruitful plateau? We will see the answer shortly.
c. Stable dry land. The third major task of this 2 billion year period was the creation of dry land. This is the task that found its way into Day Three of the Genesis Creation account.
When the earth first cooled, the earth's crust was smooth, fairly uniform, and denser than the dry land today, punctuated by frequent volcanic peaks, but as a whole, the early earth was covered by oceans.
Under the crust, heat convection currents developed in the
interior
of the earth to carry away heat from the core. Portions of the crust
fractured
into large plates, and the plates began to pile up along the fractures
as the underlying currents moved them along.
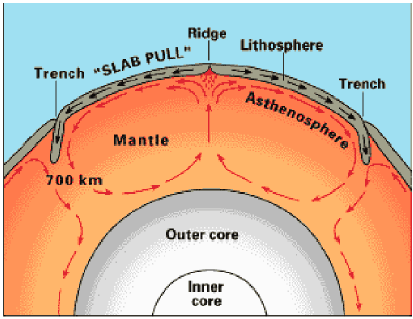 |
Convection Currents and plate movements43
The edges of the plates that end up underneath in the collision
are carried along by the mantle currents deep into the earth's
interior.
As this happens, the sinking edges of the plates melt from the heat.
The
material with a lower melting point of course melts first. It also
happens
to have a lower density, and as it melts, it rises through cracks,
leaving
heavier density matter behind. The following sketch shows this action,
which takes place, for example, along Western coast of South America,
forming
over time the South American continent and the Andes mountains.
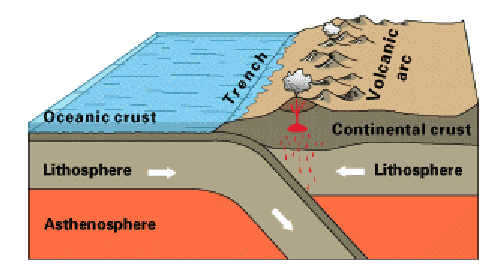 |
Subduction of the Pacific Crust44
Over billions of years, the lighter crust material rises to the surface and forms what are now the continents, composed largely of granites. Granite may seem to be pretty heavy, but it is lighter than the rest of the earth's crust, and so it tends to float on top. That's good, because in a collision with the heavier crust material the granite stays on top. This is why dry land formed in this way stays dry. The heavier crust (called magma) remains underwater on the ocean floor. Thus, today, ocean floor crust is denser than continental crust.
Eventually, there is a balance between erosion and the formation of new granite so that the overall amount of dry land remains fairly steady. This happens at around 1800 My ago.
So you see that the creation of stable dry land involved a long process of separating the minerals of the earth's crust into lighter continents that can float over the denser crust material. The earth's crust was literally pulled apart and sorted. The chemical term is fractionation. It is what a refinery does when it separates crude oil into refined products such as heating oil and gasoline.
The fitness scorecard at the end of this third period shows that atmospheric oxygen is finally at about today's level, 20-25% by content. Fixed nitrogen and organic nutrients are being produced worldwide. Dry land has almost reached its stable size.
We are moving nicely towards a fit place for plants and animals. But, as we mentioned, there is one problem: the potential for oxygen poisoning.
6. Preparation for higher life 1800-540My.
Here is the situation 1800 My ago, after 2 billion years of preparation:
a. The atmospheric radiation shield. First let's look at the atmospheric shield. Harmful radiation comes from cosmic rays - fast-moving particles from outer space - and from solar radiation, particularly radiation from solar flares. The familiar radiation badges worn by xray technicians, for example, measure radiation in rems. Generally a short-time (human) exposure of 500 rems and a lifetime exposure of 1000 rems is considered fatal. For comparison, the average background cosmic radiation from space at the top of Earth's atmosphere is about 400 rems/year.
Solar Particle events (SPEs) and Coronal Mass Ejections (CMEs)
are
huge eruptions on the Sun that can spew out energetic photons (extreme
ultraviolet, xrays and gamma rays) that can shower Earth. Occasional
Solar
eruptions may produce 200 rem/hr for periods of several days. In one
recent
measurement, 2600 rems were produced by a single SPE, as measured in
space
near to Earth. These eruptions tend to follow a cycle of Sun activity
that
today peaks about every 11 years. Currently (2001) solar activity is
near
a 11 year maximum, and SPEs are most common in the 2-3 years after such
a maximum.
This recent photograph, taken in February, 2001 shows a major
CME
in the lower right portion of the image.
 |
 |
|
|
|
Even a single day exposed to radiation from a solar event of this magnitude would be fatal to most living species.
It is evident that life on dry land without atmospheric protection, would quickly be killed by cosmic and solar radiation. This is why plants and animals do not show up on dry land until long after this period - not until about 350 My ago.
The solution that allows life to exist on dry land is the development of an ozone shield in the upper atmosphere. Ozone occurs when oxygen is zapped by cosmic and solar rays. But the ozone, once it exists, tends to react with the rays, and prevents most of the rays from reaching the earth's surface. Of course, the ozone shield could not exist until the atmosphere had a steady supply of oxygen. So when the oxygen levels stabilized about 1,800 My ago, the ozone layer began a slow build-up, and over the next billion years or so, it reached a level where it gave adequate protection to life on dry land. In the fossil record, this is about the time that the first mosses appear on land. Once it started, land life flourished and began to build up soil nutrients to support the complex plants and animals that would come later.
b. The Second Great Creation of life: Eubacteria. Bacteria, the only life forms present to this point, do not have the basic design that is needed for complex life. A new cell type is needed, so radically different that it amounts to a new, second, creation of life. The new creation is called the eukaryote cell, and occurred around 1.8 billion years ago. All plants and animals are made from eukaryotic cells, a word that means proper seed or proper nucleus.
We will first look at some of the unique features of this new kind of life, and after this we will discuss its significance.
Nucleus. The most visible distinction between bacteria (also called prokaryotes) and eukaryotes is the appearance of a nucleus - think of the yolk of an egg. You can generally identify a eukaryotic cell by the presence of a nucleus.
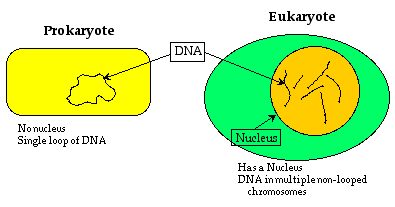
Prokaryotes and Eukaryotes
All eukaryotes have the genetic material - the DNA - in long strands called chromosomes, enclosed in a nucleus that is separated from the rest of the cell by a protective membrane. In contrast, prokaryotes - the bacteria - have looped DNA that is not separated from the rest of the cell.
The nucleus protects the DNA from damage by contact with food or cell invaders. Passage of material across the nuclear membrane is controlled by complex molecules that are designed to allow only certain kinds of material to pass.
Organelles. The nucleus is only one of a number of
specialized
portions of a eukaryotic cell. Various organelles are found throughout
the cell, each type designed to provide a local environment to carry
out
particular tasks. For example, the mitochondria are the primary source
of the cell's heat and energy. The mitochondria use oxygen to make
energy
and this is the main reason why eukaryotic cells require oxygen.
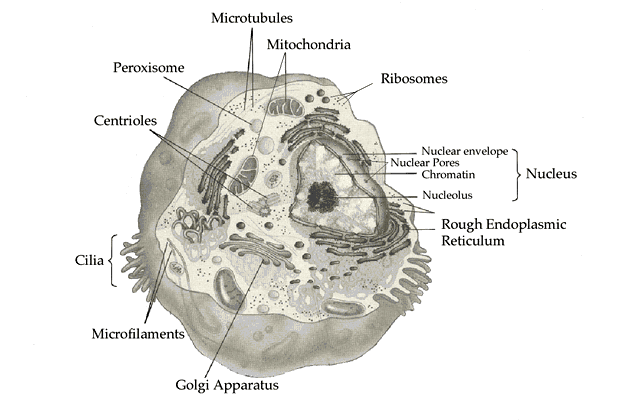 |
In addition, eukaryotic cells sometimes have other specialized features such as cilia, and a gullet.
Although there are some specialized structures in a bacterial cell (for example, some bacteria such as E. coli have flagella, which look somewhat like cilia--but are structurally quite different), overall it is a much simpler structure. In fact, some biologists think that some of the organelles in eukaryotes - specifically the mitochondria and chloroplasts - originated as bacteria and took up residence in the eukaryotic cells, eventually losing the ability to survive independently.
Cytoskeleton48
Eukaryotic
cells also have a cytoskeleton. This is an internal structure that
connects
all of the organelles and the cell wall in an intricate web of fibers.
The fibers are used for general structural support, for food transport,
and for movement, including one kind of fiber that can contract like
muscle
fiber.
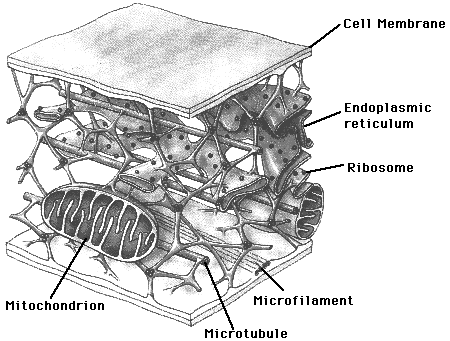 |
The Cytoskeleton -- showing filaments and
microtubules connecting cell organelles and cell walls
The filaments that make up the cytoskeleton are so fine that they are not normally visible in a microscope.
Kinesin Motor. Perhaps the most remarkable innovation in
the eukaryotic cell is the use of the cytoskeleton to form a transport
system that carries food and waste products throughout the cell. This
is
truly a motorized transport system, using a molecular motor called
kinesin.
The kinesin motor carries cargo (food or waste) in a small sack called
a vacuole, and move it along the cytoskeleton microtubules, to and from
the various cell organelles, and between them and the cell wall.
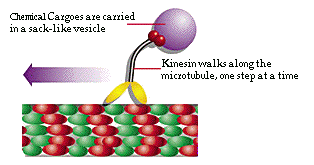 |
Kinesin Motor49
Kinesin motors were first observed by accident in 1981 by a Dartmouth professor Robert D. Allen, when he used a television camera to view the image of squid nerve fiber under a light microscope. By adjusting the image brightness, it was discovered that details could be seen that are a tenth of the size that is normally visible in a light microscope, and for the first time it was actually possible to see little round objects moving along the nerve fiber. These turned out to be kinesin.50
Signalling Mechanisms. Finally, the traffic within and between cells would be total chaos without the invention of elaborate and effective signalling mechanisms. For example, the kinesin motors require some way to know where to pick up and deliver cargo.
c. Consequences of the Eukaryotic Innovations. The innovations of the eukaryotic cell led to many practical consequences.
The nucleus protects the DNA from damage by contact with food or cell invaders. When the DNAs genes are copied prior to building the various proteins and other complex molecules of life, the genes are processed within the nucleus to remove un-needed information, and to correct copying errors.
It is hard to overemphasize how this contrasts with bacteria. The DNA of bacteria comes in constant contact with the cell contents, and as a result is subject to both random and deliberate changes in the DNA code itself. That is how viral infection works: a virus injects its genetic material into a bacterial cell, and the material then inserts itself into the cell's own DNA. From this point the cell begins to reproduce the virus, using the cell's own genetic machinery.
Bacteria are designed in this loose genetic way because one of the strong points about bacteria is the ability to respond to environmental changes by changing its genetic make-up. Bacteria can even share segments of DNA from other bacterial species, perhaps through snips of DNA that enter the cell as food. This is why bacterial species, such as the common E. Coli have so many different subspecies, both harmful and beneficial. One biologist, Lynn Margulis, argued that the concept of species is not really appropriate for bacteria because there is so much genetic variation. She notes:
†- Because bacteria that differ in nearly every measurable trait can receive and permanently incorporate any number of genes from each other or from the environment, †- the concept of "species," applicable to named eukaryotes, seems inappropriate for the Prokarya.51But for higher species, that advantage is overshadowed by the need to guard the genetic code's accuracy. The code is much more complex, and changes are very likely to be unwelcome. So for eukaryotes, the emphasis is on limiting changes in the code and controlling the accuracy when the code is copied. This work takes place within the nucleus.
Bacteria have simple balloon-like shapes (spheres, rods,
spirals)
which are determined by the way the cell wall is formed. There are just
a few basic bacterial shapes, ranging from spheres to rods to spirals.
The bacteria maintain their shape by osmotic pressure.
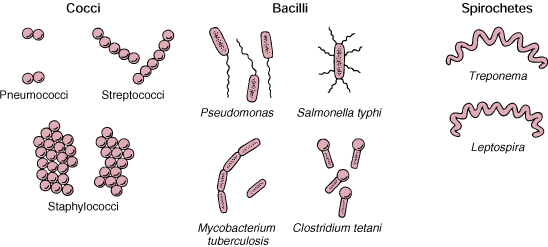 |
Eukaryotic cells have the structure and capabilities of the cytoskeleton. This allows a fantastic range of shapes and sizes. As an example, the following figure shows part of a large chart that shows different single-celled ciliates. The familiar slipper-shaped paramecium is shown center right.
|
|
In contrast, eukaryotes have an efficient internal transport system for food and waste, using kinesin and the cytoskeleton. As a result the cell size can be much larger - some ciliates, for example can be visible to the naked eye, up to 2 mm (0.1 inch). Some single nerve cells in larger animals can even be several meters long. This would be impossible without the transport system. The volume of a typical eukaryotic cell is ten thousand times larger than the volume of a bacterial cell.
On the other hand, because they are
larger, some
eukaryotic cells are less able to withstand environmental extremes such
as freezing, so size and complexity come at a price.
Gullets are specialized for engulfing food. The cilia aid in movement and also create currents that move food toward the gullet.
We already mentioned mitochondria. They
are the
power plants of the cell: they energize the cells metabolism much as
photons
provide energy in photosynthesis. They use oxygen to do this, producing
carbon dioxide as a waste product. This is the main reason why
eukaryotes
consume oxygen.
All multi-celled species, which will appear much later, after the ecosystem has been prepared, are eukaryotes. All of the new features that were introduced with the creation of the eukaryotic cell are needed to support the new requirements of multi-cellular life. In particular, the use of signalling is carried to new heights of development in inter-cellular communication and especially in embryo development. These are topics that go well beyond the scope of this talk, except to note that the seeds of these new features are found in the new capabilities of the eukaryotic cell.
At the start of this period, life on earth went through continual cycles of boom-and-bust. Some of these cycles were the result of natural disasters such as meteorites or ice ages.
But there was a basic instability that was caused by the nature of bacteria itself. There are three factors to consider:
But once the material in the oceans and earth's crust were largely oxidized, there was no longer a natural way to remove excess oxygen. Thus there was the potential for life to reach a sickly stagnation caused by its own waste.
The creation of eukaryotes at this point solved the dilemma by providing an essentially limitless sink for the oxygen. Oxygen was recycled back into carbon dioxide by the mitochondria, and so eukaryotes and bacteria could both thrive, each domain of life supplying the food and consuming the waste of the other.
Thus the creation of a new kind of life,
and
at this stage in the preparation of the ecosystem, was necessary for
the
preparation to proceed. This creation occurred about 1,800 My ago (give
or take a few hundred million years).
Eukaryotes on the other hand, (see the
many varieties
of ciliates, for example) can be highly efficient foragers, and their
larger
and more specialized features make them able to engulf and digest food
(bacteria and other eukaryotes) with much greater efficiency.
The cytoskeleton structure of the eukaryotic cell supported the development of new mobility, leading to a greater ability to migrate and avoid dead zones (caused by a lack of nutrients or a buildup of wastes).
For the next billion years, the first eukaryotic species spread through the earth, achieving a critical balance with bacteria and adding to the store of nutrients that will be needed by the more complex plants and animals.
This period ends at the so-called Cambrian explosion, which was a period of great creative intensity, lasting perhaps as little as 5-10 My, during which most (some say all) of the modern phyla of life appear in the fossil record. It is marked in the fossil record as the first appearance of multi-celled species that have skeletal parts. We will begin at this point in a future talk.
At the end of this period, a fitness scorecard indicates that the earth is now a fit place for plants and animals. There is still no significant life on dry land, but the ozone layer filters most harmful rays from the earth. The earth is filled with organic nutrients. Every product of one species of life is food for another species. The earth is ready to host stable and enduring multi-cellular life.
7. Why would God do it this way?
Why would God create the world in this long, involved way? I think that part of the answer is that God wants us to learn the details of his work by examining his Creation. He loves to reveal his handiwork, as Psalm 19 states.
I believe that God wants mankind to learn the capabilities and limits of natural development. He does this by creating the world using natural processes whenever those processes will do the job. He wants us to learn about these natural processes so that we can learn both the power and the limitations of nature. The fact that some of these natural processes took billions of years is not a problem with God, because he is not limited by time and space.
You might ask, Why didn't God create a world that could do everything naturally? Why build something that required him to intervene? Perhaps one answer is that such a world would have the attributes of God himself. We already hear scientists and philosophers who attribute god-like powers to nature. Mostly, I think, this attribution is based on lack of full information: when you don't know the details and time scale of some feature in the history of living species, it is easy to say that it must be easy to do, or that it can be done by familiar natural, undirected forces. As your knowledge increases, the second argument sounds less plausible, and so the first becomes the argument of choice, even though we don't know how to do it. But of course, this is an argument from ignorance, or else it says in essence that Nature has divine qualities.
I suspect that the nature of the Christian faith is such that there will always be contention with secular science, however enlightened both sides become to the issues. It is absolutely basic to the Christian faith that God actively participates in his creation. We may not know some of the details, but the fact of God's interest and involvement is basic to our faith.
8. Why didn't I look into these things years ago?
For me, this talk forges new territory. For years I failed to look seriously into the claims of evolution, geology and the fossil record. I knew of course that the common secular view is that these things abundantly prove the theory of natural evolution.
Perhaps I feared that there is some fatal flaw in my understanding of how God works, and studying these things would shake my faith. The foes seem so strong and noisy when it comes to evolution and the implications for a godless world view. Better, maybe, to stay ignorant and even wrong about these things, in order to protect my faith.
Well, by God's grace, my scientific curiousity got the better of me, and I discovered that there is no reason to be defensive when it comes to such matters. Here is how I approach secular science.
I try to distinguish between objective, experimental facts and the secular interpretations of the facts. I believe that God has put such a level of basic curiosity into the human soul that even an atheist cannot totally distort the drive of his curiosity.
From a lifetime of scientific work, I have come to believe that we can trust the modern scientist when he is doing empirical scientific investigation. Not that he is necessarily right, but because every true scientist opens himself up to critical evaluation. Science is highly competitive and the key for success is demonstrable proof. This means that it must be possible to verify facts of science by independent investigations of people who do not have an axe to grind.
I definitely do not subscribe to a conspiracy theory about science. Oh, sure, there are those noisy atheists who will try to interpret scientific facts to prove their atheistic position, but science as a whole is not conspiratorial: it is too much of a free exchange to be that. Perhaps evolutionary biology or psychology come the closest to exercising control over the standard view, but in the long run, most scientists understand that such thought control is self-defeating. There are too many times that the politically correct ideas prove later to be wrong.
So I get very distressed by people who hold to a brand of creation science that is radically different from what you hear from established scientists. Not that they hold different views, but because they imply that the only reason their views are not accepted is because of some conspiracy against those views. I have not found this to be so.
Let me put this bluntly: if it is indeed true that radically different interpretations of science are the correct ones, then objective scientific proof will smell it out. No scientist can get away with dogmatic claims that fly in the face of impartial objective evidence. That is the marvel of that little itch of eternity that God has placed in every man's heart.
The development of a fit place for life can
be
summarized in the following fitness scorecard. The requirements are
those
discussed in this talk, and the time periods correspond with the
different
stages discussed here.
|
(Million Years before Present) |
||||||
|
"A Fit Place for Life" |
"Abundant Life" |
|||||
| Requirement | 4550 to 3800 |
3800 to 3400 |
3400 to 1800 |
1800 to 600 |
600 to 450** |
450 to Now |
| Mixing (tides/storms) |
Yes | Yes | Yes | Yes | Yes | Yes |
| Liquid Water | Yes (at end) |
Yes | Yes | Yes | Yes | Yes |
| Available Nitrogen |
Some | Yes | Yes | Yes | Yes | Yes |
| Oxygen Atmosphere |
No | No | Yes (at end) |
Yes | Yes | Yes |
| Organic Nutrients |
No | No | Some | Yes | Yes | Yes |
| Dry Land | No | No | No | Yes | Yes | Yes |
| Radiation Shield (Ozone Layer) |
No | No | No | Buildup | Yes (at End) |
Yes |
| Complete Recycling |
No | No | No | Yes | Yes | Yes |
| ** First Land Plants (algae) and animals show up 450 to 400 Million years ago. | ||||||
References
Note: All references, except as noted, are written from a secular viewpoint. Most of these books gush unnecessarily about the specious Miller-Urey experiments, but perhaps that is one of the necessary sops that one must throw to the evolutionists.
Wallace S. Broecker, How to Build a Habitable Planet, Eldigio Press, 1985.
This is a remarkably readable discussion of the preparation of the earth for habitation. It covers in greater depth some of the topics mentioned here, as well as many others.G. Brent Dalrymple, The Age of the Earth, Stanford U. Press, 1991
This book shows how the age of the Earth, the Moon and the Solar System is obtained from dating of Earth rocks, Moon rocks and meteorites. It is a comprehensive (as of 1991) but readable account. Chapter 9, "What We Know and Do Not Know"is particularly helpful.Michael J. Denton, Natures Destiny: How the Laws of Biology reveal Purpose in the Universe, Free Press, 1998.
Fascinating and readable account of the problems that recent findings in biology present to those who believe in undirected natural evolution. Extensive discussion of the uniqueness of biological materials.Boyce Rensberger, Life Itself: exploring the Realm of the Living Cell, Oxford, 1996.
Very readable treatment of basic cell processes. Many photographs and diagrams showing the function of the cytoskeleton, molecular motors, mechanisms for cell movement, etc. Useful despite strong evolutionary bias.Hugh Ross, Design and the Anthropic Principle, Reasons to Believe. Extracts are located at http://www.reasons.org/resources/under the topic "Evidence for Design"
Hugh Ross has many books written from the viewpoint of a Christian scientist, defending the Creation viewpoint. His arguments for design are far-ranging, scientifically solid and well documented. His works are valuable, quite readable, and thought-provoking.William H. Schlesinger, Biogeochemistry: An Analysis of Global Change, Second Edition, Academic Press, 1997
This book gives detailed descriptions of the various biological cycles in the ecosystem (carbon, oxygen, nitrogen, sulfur, etc.), how they originated, and how they are kept in balance.J. William Schopf, Cradle of Life: The Discovery of Earth's Earliest Fossils, Princeton, 1999.
An excellent and readable book about Schopf's discovery of the earliest fossils. The book is particularly valuable in its careful description the scientific evaluation of the fossils, and how the age and identity of the fossils were accepted in the face of substantial skepticism. A chapter on the so-called Martian fossils is also included.Peter D. Ward and Donald Brownlee, Rare Earth: Why Complex Life is Uncommon in the Universe, Copernicus Press (Springer-Verlag) 2000.
Excellent discussion of the many unique features of the Earth that lead to the Rare Earth Hypothesis. Unfortunately, the authors assume that simple life is common in the universe, an argument (Chapter 1) that entirely overlooks the complexity of even the simplest life forms, much less the extremophiles (Archaea) that the chapter discusses. Fortunately this assumption is totally irrelevant to the book's thesis.Roger C. Wiens, Radiometric Dating: A Christian Perspective, A resource paper of the American Scientific Affiliation and the Affiliation of Christian Geologists. Available at
Excellent discussion of geological dating methods.Christopher Wills and Jeffrey Bada, The Spark of Life: Darwin and the Primeval Soup, Perseus Publishing, 2000.
Interesting discussion of the history of biological interest in the origin of the planet earth and of life. An evolutionary perspective, but much useful information.W. M. White, Isotope Geochemistry, Cornell University Lecture notes, 1998, available at:
This is an advanced presentation of how geological ages are found from analysis of isotopes. Zircon dating is lecture 9.
1 Hebrew
tohu
= empty, barren, waste. The same word appears in Genesis 1:2 "Now the
earth
was without form and void" (KJV) "formless and empty"
(NIV)
"a formless void" (NRSV).
2 Some earlier
talks in this series have been published as IBRI Research Reports,
available
at http://www.ibri.org: RR#49,
A
Physicist Looks at Creation day One, RR#50, The
Chemical Building Blocks of Life. One lecture in the
series
has not been published as a Research Report but the original recording
is available from IBRI as IC-003, The Right Chemistry for life.
3 See
http://www.arthurhu.com/index/evol.htm
for a comprehensive chronology offered by Arthur Hu. His web site is a
useful (somewhat idiosyncratic) source for remarks on various
creation/evolution
topics, and includes many cross-references to other sites. For more
conventional
chronologies see: Niel Brandt's table at http://www.talkorigins.org/origins/geo_timeline.html.
4 See the CRC
Handbook of Chemistry and Physics, table on bond strengths of
diatomic
molecules. The nitrogen bond strength (225.9 kcal/mol) is nearly double
the strength of oxygen (119.1 kcal/mol) and most other common
molecules.
This bonding energy is one reason why explosives are typically
nitrates.
See also http://www.ultranet.com/~jkimball/BiologyPages/B/BondEnergy.html.
5 Research
results
on solar wind and cosmic radiation are available on the internet in
connection
with a manned Mars landing at http://www.seds.org/pub/info/mars/RadHuman.txt.
See also Lisa C. Simonsen and John E. Nealy , Mars Surface
Radiation
Exposure, NASA TP-3300, February 1993, available at: http://techreports.larc.nasa.gov/ltrs/refer/93/rdp3300.tex.refer.html.
6 See G. Brent
Dalrymple, The Age of the Earth, Stanford U. Press, 1991,
Chapter
3,"Modern Radiometric Methods". This book was written explicitly to
address
the objections of Creation Science proponents. See also Roger C.
Wiens,"Radiometric
Dating: A Christian Perspective," a resource paper of the American
Scientific
Affiliation, available at http://www.asa3.org/ASA/resources/Wiens.html.
7 Reproduced
from
http://anthro.palomar.edu/time/table_of_isotopes.htm
with permission by Dr. Dennis O'Neil.
8 There is
another
use of radioactive measurements in connection with biological materials
(for example in carbon-14 dating), but we will not consider that use in
this talk.
10 For
accounts
of this period see Wallace S. Broecker, How to Build a Habitable
Planet,
Eldigio Press, 1985, Christopher Wills and Jeffrey Bada, The Spark
of
Life: Darwin and the Primeval Soup, Perseus, 2000, and Peter D.
Ward
and Donald Brownlee, Rare Earth: Why Complex Life is Uncommon in
the
Universe, Copernicus Press, Springer-Verlag, 2000.
11 G. Brent
Dalrymple,
op.
cit. Table 6.3.
12 From a
Gallery
of images at http://www.psi.edu/hartmann/.
These Images are used by permission of William K. Hartmann.
13 This
scenario
is described in Peter D. Ward & Donald Brownlee, op. cit.,
p229ff.
14 Ward and
Brownlee,
Op
Cit. Fig. 5.12 and Table 5.6
15 Ward and
Brownlee,
Op.
Cit., p. 227.
16 The rate of
recession is actually faster today than it has been at times in the
past,
for complex reasons related to the distribution of mass in the earth.
17 Dalrymple,
op.
cit, p. 225.
18 Wills &
Bada, p77. They do not indicate how the ages of the younger craters
were
determined.
18a A good
map
of the moon is located at http://www.oarval.org/MoonMapen.htm
19 Alphonsus
photographs from http://nssdc.gsfc.nasa.gov/imgcat/midres/ra9_a035.gif
and
http://nssdc.gsfc.nasa.gov/imgcat/midres/ra9_a060.gif.
Public domain.
20 From
http://antwrp.gsfc.nasa.gov/apod/image/orientale_lunar4.gif.
Public domain.
21 Ward and
Brownlee,
Op.
Cit.
22 Vapor
pressure,
which varies for all liquids and gases, causes a close connection
between
an atmospheric gas and the amount of the gas dissolved in water,
because
there is a constant gas exchange at the surface. Oxygen is not very
soluble
in water, but nonetheless its presence in the upper levels of the
oceans
is vital for the existence of higher species of aquatic life. So, if
the
atmosphere did not have oxygen, neither did the ocean waters.
23 Indeed, the
scientists who found the Martian fossils appear to have been misled by
such features. See J. William Schopf, Cradle of Life: Discovery of
Earth's
Earliest Fossils, Princeton, 1999, Ch. 12.
24 Schopf,
op.
cit., p. 88.
25 See
composite
photograph in Schopf, op. cit. Fig. 3.4. A similar composite
is
found at
http://www.astrobiology.ucla.edu/ESS116/L15/1515%20Apex%20Chert.jpg
26 Photograph
from From http://www.biol.tsukuba.ac.jp/~inouye/ino/cy/36.gif.Used
by permission. For discussion of Anabaena Cyanobacteria see Lynn
Margulis
and Kathlene V. Schwartz, Five Kingdoms: An Illustrated Guide to
the
Phyla of Earth,Third Edition, W.H. Freeman, 1999, p79.
27Photographs
at Hamelin bay, courtesy of Jo Peters and Martin W. Peters, Landmark
Solutions,
at http://www.landmarksolutions.com.au. Used by Permission. For
another view of living stromatolytes at Hamelin Bay, see http://www.discoverwest.com.au/strombig.JPG
28 Both
charged
(alpha and beta rays) and uncharged (gamma) cosmic rays and solar rays
constantly bombard the Earth. Charged rays are deflected by the Earth's
magnetic shield, but at this date (2001) the development of the
magnetic
shield throughout Earth's history is only partly understood. Uncharged
rays (gamma rays) occur over a wide range of energies, some exceedingly
high, but with the majority in the far ultraviolet range about 4-5
times
the energy of visible light, an energy just below soft xrays. 23.073
kcal/mol
= 1 electron volt. A "mole" of cosmic rays is 6.02 x 1023 separate
particles.
See Harold J. Morowitz, Beginnings of cellular Life, Yale
University
Press, 1992, Table 12, p.124. Also see
http://www.seds.org/pub/info/mars/RadHuman.txt.
[note July 2003: Students for the Exploration and Development
of
Space (SEDS) does not appear to be an active
site.]
29 Morowitz,
op.cit.
Table
12.
30 Margulis and
Schwartz,
op
cit., p48 state: "Probably the most important evolutionary
innovation
on Earth, if not in the solar system and the galaxy, was
photosynthesis,
the transformation of the energy of sunlight into usable form." Colin
Tudge,
The
Variety of Life, Oxford, 2000, p.125 states:"Photosynthesis is a
tremendously
intricate process that depends on the molecule chlorophyll in its
various
forms, and it seems unlikely that such a molecule could have originated
repeatedly."
31 For a list of the
chlorophyll genes see http://reith.imb.nrc.ca/ct.htm.
In cyanobacteria the chlorophyll occurs in a membrane called the
thylakoid
membrane (see the figure of anabaena). The ATP Synthase molecule is
embedded
in the membrane.
32 We will use the
terms hydrogen ion and proton interchangeably. Technically, a heavy
hydrogen
ion is not a proton, but heavy hydrogen ions do not work well in
photosynthesis.
The proton current is maintained in a "proton pump"in which water
molecules
"pass along" the protons in a rapid cascade that may be diagrammed as
follows:
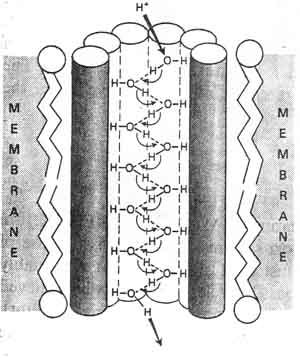
 You
can contact IBRI by e-mail at: webmaster@ibri.org
You
can contact IBRI by e-mail at: webmaster@ibri.org
Last updated: July 18,
2003;
Hyperlinks updated 21-Jul-2003