1.4 Classical Mutation Theory vs. Neo-Darwinian Evolution
1.4.1 Mutation. When Hugo de Vries postulated the term mutation, he believed that mutation alone can bring about the abrupt changes of genetic constitution. He divorced evolution from natural selection by maintaining that natural selection merely has the negative effect of "pruning" the genetic varieties that are unfit to survive and thus plays no role in the diversification of genic variation that is the essence of evolution. This has been known as the classical mutation or saltation theory.
De Vries's contemporary W. Johannsen (1857-1927) added impetus to the classical mutation theory by demonstrating that variation in the size of garden beans did not respond to the effect of natural selection (1). From a seed lot of a single variety he selected out the largest beans and the smallest ones. He found that only in the first generation did the cross between plants grown from the larger beans produce slightly larger beans than a cross between plants grown from smaller beans. In the subsequent generations, the selection of the size of the bean during mating had no effect on the offspring. He concluded that natural selection had no effect on the fluctuating variations Darwin observed in the natural populations. Other geneticists who supported the mutation theory thereby stressing the importance of the sudden origin of discontinuous variations as a source of evolutionary change were William Bateson (1861-1920) (2) and [43] S. I. Korzhinsky (1861-1900) (3). Their criticisms dampened the enthusiasm of the Darwinists, who believed that natural selection was the major driving force for evolution.
The concept of mutation was later elaborated by the Nobel laureate Thomas Hunt Morgan (1866-1945) and his associates, who refined de Vries's conclusion by their experiments with the mutability of the fruit fly Drosophila melanogaster (4, 5, 6). They showed that the effects of the mutations that occurred in the flies were of varying degrees of severity. The effects ranged from ones so drastic that the mutants were lethal to moderate effects to those barely detectable. Mutants with drastic changes were easily recognized by an untrained eye, and they were most useful in genetic analysis. They reexamined de Vries's data and found that the mutants he obtained were actually an assemblage of diverse mutations that appeared to be drastically different from its parental plant. These findings led de Vries to conclude that sudden mutation gives rise to new species that he understood to mean a new line of pure-breeding genetically identical individuals.
Morgan's work was later elaborated by his student, Nobel laureate H. J. Muller (1890-1967) who related the frequency of mutation to the effects of radiation (7, 8, 9). He measured the frequencies of certain classes of mutations and showed that they can be increased experimentally, i.e., by the effect of x-irradiation. His work also stimulated the search for other mutagenic radiations and chemicals. The work of Morgan, Muller, and their colleagues paved the way for the Neo-Darwinian view that mutation provides the raw material for evolution.
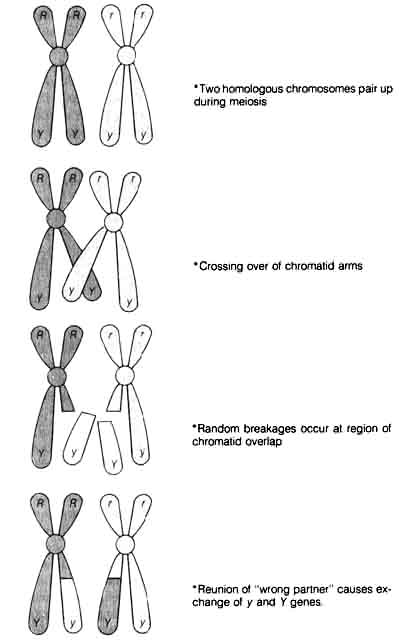
1.4.2 Recombination. A further source of diversity, namely, recombination, was also discovered and later contributed directly to the Neo-Darwinian view of the origin of variation in the living world. William Bateson, Reginald Punnet (1875-1967), and their associates first observed the departure from the Mendelian ratio expected from independent assortment in crosses between different varieties of sweet peas (10). They found that parental plants of different flower color and pollen shape gave rise to F1 and F2 offspring in which the genes for flower color and pollen shape did not assort independently. These genes seemed to be tied together (later termed linkage) so that the F2 offspring showed ratios of too many of the original parental genotypes and too few of the newly combined genotypes. The correlation of the Mendelian paired factors (alleles) with homologous chromosomes that paired up during meiosis, shown by W. S. Sutton (1877-1926) (11) and T. Boveri (1862-1915), (12) clarified the mechanism of this unexpected genetic phenomenon. Furthermore, Morgan helped clarify the picture by his study of sex-linked characteristics in

Figure 1.5. Schematic model of crossing over. c: centromere that connects chromatids on the chromosome R, r, Y, y genes; same designations as those of Figure 1.2.
[45] Drosophila melanogaster (13).The
characteristics that did not assort independently during meiosis were located
on the same chromosome. Therefore, during the segregation process, they
stayed together. As a result, the genotypes of the Fa comprised a majority
of the parental types while the rare newly combined genotypes arose by
the process of crossing over or recombination. These events are illustrated
in Figure 1.5.
With these added features of the genetic explanation of variation, the classical theory was slightly modified. For the classicist, variation arising from mutation and recombination is removed by the purifying force of natural selection that rejects all but the fittest type. This is the so-called stabilizing selection. (see I. 1.4.4). Therefore, natural selection was seen as antithetical to variation, and a genetic basis for evolution was unsubstantiated.
The theory of evolution via natural selection was held at low esteem between 1900 and 1925. The position of many evolutionists during this period can best be represented by the following excerpts from William Bateson's speech given at the 1921 convention of the American Association for the Advancement of Science (14):
I may seem behind the times in asking you to devote an hour to the old topic of evolution. Discussions of evolution came to an end primarily because it was obvious that no progress was being made . . . . When students of other sciences ask us what is now currently believed about the origin of species, we have no clear answer to give. Faith has given place to agnosticism . . . we have absolute certainty that new forms of life, new orders and new species have arisen on earth. That is proven by the paleontological record . . . our faith in evolution stands unshaken. (Italics mine)1.4.3 Agnostic Period. Although the mechanism of evolution by natural selection fell into disrepute, evolutionists clung onto their faith by relying on the circumstantial evidence of the fossil record. Edward O. Dodson and Peter Dodson named this period "The Agnostic Period" of development of modern evolutionary thought (15). Although the classical mutationist position was gradually replaced by the modern dominant view of Neo-Darwinism, it was recently revived by the advent of the field of molecular evolution (a study of evolution by the modern techniques of molecular biology) in the form of neutral mutation theory that R. C. Lewontin called neo-classicist (16). We shall return to the discussion of the neutralist-selectionist debate in a subsequent section (see I.3.3.2.a.1).
The restoration of Darwinian natural selection as the principle guiding factor in evolution began when J. B. S. Haldane (1892-1964), R. A. Fisher (1890-1962), S. Wright (b. 1889), and S. S. Chetverikov (b. 1880) independently worked out the theoretical models to study the variations in [46] population. However, the modern synthetic version of the Neo-Darwinian theory was not formulated systematically until the publication of Dobzhansky's Genetics and the Origin of Species in 1937 (17). Theodosius Dobzhansky (1900-75) correlated mathematical models in population genetics with the refined chromosomal theory of heredity by the Morgan school. Dobzhansky's work was supplemented by writings of J. S. Huxley (b. 1887), E. Mayr (b. 1904), G. G. Simpson (b. 1902), and G. L. Stebbins (b. 1906), all of whom attempted to present a strong case for the synthetic theory of evolution.
For the Neo-Darwinist, evolutionary changes take place when the gene variation by mutation and recombination is subject to the process of natural selection. These changes are determined at the population level by the way in which the environment is changing relative to the adaptation of the organisms in the population. Based on the different organism-environmental interactions, natural selection seems to be operating in three different ways, namely, stabilizing selection, directional selection, and disruptive selection. These are depicted in Figure 1.6.
1.4.4 Stabilizing Selection. Stabilizing selection (normalizing selection) eliminates any marked deviations from an already well-adapted population. It is essentially the type of selection referred to by the classicists. But contrary to the classicist's view that this was the only role of natural selection in evolution, Neo-Darwinism maintains that stabilizing selection is only one of several ways natural selection can work in evolution.
An example of stabilizing selection can be seen in the predator-prey relationship of owls and field mice. Mice with normal color are protected from owls because the field is the color of the mice. However, mice with deviant color are quickly eliminated from the population because they are more visible to owls. Thus, selection tends to maintain the color of the mice within a narrow range that is determined by the color of the field.
1.4.5 Directional Selection. Directional selection is the force that drives the population to undergo evolutionary changes in one direction with respect to certain adaptive characteristics. Here deviants are not always eliminated as in the case of stabilizing selection. Deviants from the norm in one direction tend to survive more often and leave more offspring than deviants in the opposite direction.
Directional selection can most easily be observed when a population is subjected to a progressive change in environment. The most famous example of directional selection is industrial melanism as seen in the peppered moth and is discussed in detail in I.3.2.1.b.
1.4.6 Disruptive Selection. Disruptive selection has a somewhat opposite effect from stabilizing selection. The latter favors homogeneity of a [47] population by eliminating extreme variants, whereas disruptive selection tends to eliminate the majority of a population and establish the extreme variants.
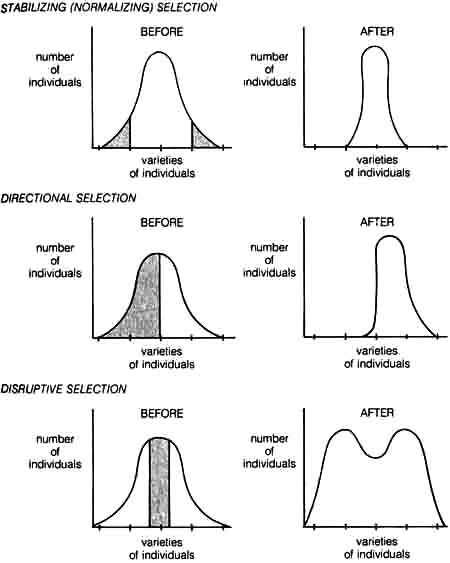
Figure 1.6. Diagrams illustrating the effects of stabilizing (normalizing), directional, and disruptive selection. Varieties of individuals can be represented by phenotypic variations such as height, skin color, etc., that are controlled genetically. Shaded and open areas on the Before selection curves represent adverse and favorable selections respectively.
[48] An example of disruptive selection is seen in the development
of the seed size of some plants when certain beetles specialize in feeding
only on intermediate-size seeds. The result is the elimination of seeds
of intermediate size and the plants producing the intermediate seeds. The
outcome is two distinct populations of plants. One population has small
seeds and the other large seeds.
References 1.4
1. Johannsen, W. Elemente der exakten Erblichkeitslehre. Jena: Gustav Fischer; 1909. Reprint; 1926.