[55] Evidence for Evolution
2.1 Antiquity of the Earth
2.1.1 Principle of Uniformitarianism. Since evolutionary processes require long periods of time, the establishment of the antiquity of the earth through geology is a prerequisite for the credibility of the evolutionary theories. Geologists concerned with estimating the earth's age use fossils as one source of data.
In medieval times fossils were thought of as a product of some plastic force in the earth, or unusual concretion, or curiously figured stones. Leonardo da Vinci (1452-1519) was the first person to make some sense out of the curiosity of fossils. He believed fossils he found in northern Italy had been formed on the sea floor by burial of living animals in silt and mud. In 1517 Fracastoro's thinking was similar, and he concluded that the fossils at Verona, Italy, were produced by natural burial of shells. He also dismissed the Noachin Deluge as a possible cause of these fossils. Fracastoro believed a temporary inundation such as the Flood would have scattered the shells rather than bury and preserve them (1).
René Descartes agreed in principle with Fracastoro in his Philosophiae Principia published in 1644. He argued that the "laws of nature" should be used to trace the origin and progress of the earth (2). Also in agreement with Fracastoro was Robert Hooke. In his work published posthumously in 1705, he maintained that the Noachin Flood was not adequate to explain the voluminous sediment and the fossils it contains (3). He suggested that natural phenomena should be used to explain the changes in the earth. Hooke was also the first to recognize the possibility of establishing a chronology of the earth's history from the fossil record, though he did not elaborate on the details.
[56] After studying the earth's surface, George de Buffon pointed out the power of rivers and currents in the erosion of lands over a long period. He also suggested on the basis of fossil findings that the earth has not always been as it is at present and that the positions of the land and seas have changed. In 1778, Buffon attempted to arrange earth history into six long indeterminate intervals of time in his book Epoques de la Nature. The careful documentation of his novel ideas won acceptance among his contemporaries.
a) Modern Geology. Modern geology, however, was not brought into being until the principle of uniformitarianism had been systematically worked out by James Hutton (1726-97) in his Theory of the Earth published in 1795. He assumed that the past history of the earth can be explained only by what is observed or recorded to be happening at present or during the immediate past. Therefore, according to Hutton, the present must be the key to the past (4). Charles Lyell (1794-1875), the father of modern geology, built on Hutton's foundation and expounded the view of uniformitarianism in his epoch-making thesis Principles of Geology as follows (5):
All past ages on the globe had been brought about by the slow agency of existing causes. The imagination was at first fatigued and overpowered by endeavoring to conceive the immensity of tine required for the annihilation of whole continents by so insensible a process; and when the thoughts had wandered through those interminable periods, no resting place was assigned in the remotest distance . . . . Such views of the immensity of past time, like those unfolded by The Newtonian philosophy in regard to space, were too vast to awaken ideas of sublimity unmixed with a sense of our incapacity to conceive a plane of such infinite extent.b) Historic Geology. One theory after another was developed to explain the appearance of the earth's surface. The opposing view of historic geology was first presented by Baron George Cuvier (1769-1832). He believed catastrophes and restorative creations were the explanation of the fossils in the geological timetable (6). Twentieth-century catastrophists are found among the followers of Velikovsky (7), who proposed that the irregularities in the solar system's orbit indicated that the earth was involved twice in global upheavals. His ability to assemble great masses of ancient records suggestive of past catastrophes drew much attention despite his untenable theories (8, 9).
Flood geologists (10) also believe that the sedimentary deposits of the earth were caused by a catastrophe, namely, the universal Deluge. They express this belief as follows: "A tremendous cataclysm of water pouring down from the skies and up from the subterranean deeps, produced [57] a year long debacle of erosion and deposition of sediments that could have accounted for at least most of the sedimentary deposits in the earth's crust" (11).
c) Contemporary View of Uniformitarianism. Uniformitarianism is favored among most contemporary geologists for essentially two reasons: (1) It has the merit of simplicity. Since rocks can be interpreted using the physical and chemical processes observable in the present, physical geologists can develop hypotheses and subject them to verification by observation. (2) Uniformitarianism also takes into consideration the occasional catastrophes experienced by the earth. It is evident that geologic processes have acted in the same way throughout earth's history but not always with the same intensities. Some catastrophic events in the earth's history can be analyzed by their present-day counterparts, such as earthquakes and volcanic eruptions. Others, such as the Noachin Flood, can be examined only by comparing the modern processes of sedimentation with the geological record. (This comparison has led most geologists to seriously doubt the validity of the Flood geologistsí claim that most, if not all, of the earth's sediments were deposited during the temporary inundation of the Flood (12)).
There are still many questions for which advocates of uniformitarianism have not found satisfactory answers. For example, the origin of the magma (the molten material underneath the earth's surface), the formation of plutonic rocks (rocks formed within the earth's crust presumably by the crystallized magma), and the forces of mountain building are not understood. Unfortunately, these phenomena are concealed from observation and cannot be analyzed (13). Nevertheless, uniformitarianism has been the foundation of modern historical geology and is a helpful guide in deciphering physical geology, but it is a concept that requires some comprehension of geologic time.
2.1.2 Dating of the Geological Column. There are two methods of dating the geological column, namely, relative dating which gives only he order of events, and absolute dating (finite dating), which measures the duration of time from a fixed reference point (14).
a) Relative Dating. The main method of relative dating makes use of fossils. One of the first attempts to classify geologic time in terms of the presence or absence of fossils was made by J. G. Lehmann (1719-67) in 1967. He called the oldest layer of rocks that did not contain any fossils the Primitive Class. The fossiliferous rocks formed by secondary processes from the Primitive Class were named Secondary Class. The third amd most recent layer of rocks was the Third Class and contained abundant organic life. [58]
The systematic application of fossils to date rocks was not widely used until George Cuvier (1769-1832, an antievolutiemist), Alexandre Brongniart (1770-1847), and William Smith (1769-1839) independently came up with comparable geological ages, using fossils in France and England. They have shown that layers of comparable age in different places have similar fossils, with different fossils in layers above and below. Cuvier and Brongniart recognized ancient (extinct) and modern (living) fossils and used them to subdivide into groups the rocks where they occurred. These approaches were later clarified by Lyell (15). He classified European fossiliferous rocks into periods and groups in a form similar to the modern geological column.
In dating the geological record by fossils, two fundamental laws were used. The first is the law of superposition and states that undisturbed strata are in the order of their deposition of formation. The justification for the law is obvious, for the deeper layers must be formed earlier than the more superficial layers by all observable processes of rock formation. The second law is the law of faunal and floral succession and stipulates that plants and animals have progressively changed through time and that each period of geological time is characterized by distinctive fossil groups. This is verified by the observation that each geological period has a unique array of fossils and comparable ones can be located in a similar geological period throughout the continents. The nonrecurrence of these species has been a major advantage for the paleontological "clock."
Certain fossils called index or guide fossils are found to be very useful in correlating geologic time. These fossils must have the following characteristics: wide geographical distribution, ecological tolerance, abundance, and rapidly changing morphological features (16). Most paleontologists, however, prefer to work with a collection of fossils because even if index fossils are missing, the chances of correlating the last and first appearance of certain fossils is increased. However, some scholars believe the use of fossils to establish the geological time scale or vice versa is circular reasoning. The charge of using circular reasoning in the relative dating method (17, 18) is not justified in light of the above generalizations and also when the more quantitative techniques of absolute dating are considered. Attempts are made also to correlate relative dating with absolute radiometric age (19).
b) Absolute Dating. There are several methods for the estimation of absolute or finite age of rocks on the earth. The older techniques were quantitative and used physical and chemical parameters applicable to geological formations.
One method used to estimate the earth's age deals with the salinity of [59] the oceans. It is assumed that the oceans were originally fresh water and that the salt content was derived from the progressive erosion of rock salt carried into the ocean by way of rivers and streams. Fresh water from the ocean was constantly distilled by the heat of the sun and returned to the environment in the form of clouds, rain, and snow. During this circulation, it is assumed, the salinity of the ocean is directly proportional to the amount of salt carried from the rivers and streams. If the rate of transport of salt and the absolute salt content in the ocean were known, the age of the ocean, which would presumably correspond to the age of the earth, could be calculated. The figure of 100 million years was obtained by this calculation. However, since no reliable methods are available to estimate whether the amount of water that was lost by evaporation from the oceans equaled the influx from the rivers and streams, this estimate is deemed unreliable (20).
The rate of accumulation of sedimentary rocks is also used to estimate the age of sedimentary deposits of the earth. The thickness of the deposit divided into the rate of accumulation would give the time for the whole deposit to have occurred. The limitation of this scheme is the difficulty involved in determining the total thickness of continuous deposition. Also, the rate of deposition seems to vary from place to place and from time to time, being faster during episodes of mountain building and slower in flooded lowlands. Therefore, the figure estimated by this method of 95 million years since the Cambrian period was not accurate (20). This system has proved useful only in special situations when thin layers of deposit mark a given length of time that can he confirmed by radiometric dating.
The assumption that the earth has gone through a period of cooling off from its original molten state without gaining heat from sources other than the sun led Lord Kelvin (1824-1907) to calculate the age of the earth as between 25 to 100 million years. However, he qualified his estimate by assuming that it was valid only if no other internal source of heat was present in the earth's history. The estimate became meaningless when heat was found to be produced during radioactive decay in minerals found in the earth's mantle and crust.
The discovery of radioactivity provided geochronological studies (chronology of the earth based on geological data) with a powerful tool. There are five conditions that have to be met before radioactivity can be applied to geochronology (21, 22).
(1) The parent atom A by a random process becomes radioactive.Since radioactive dating is the most quantitative method of geochronological studies and has been severely criticized by some (23, 24), an extensive elaboration of principles is in order.(2) The parent atom A, by radioactive disintegration is transformed (decays) to a daughter atom B. [60]
(3) The rate that A is decaying into B is a constant with an accurately known parameter.
(4) The system of the parent and daughter atoms must remain closed since the formation of the system, e.g., time of crystallization of volcanic rock. There must be no exchange of parent and daughter atoms with the surroundings during this period.
(5) The sample analyzed is representative of the geological formation analyzed.
(1) Nature of Radioactivity. An atom consists of several types of particles. The best understood are the electrons, protons, and neutrons. Electrons revolve around the nucleus in electron shells and are negatively charged. Protons are positively charged, and neutrons are neutral, but both are bound by nuclear forces in the nucleus of the atom. The mass of the electrons is negligible as compared to that of the protons and the neutrons that have similar mass. Therefore, the mass number of an atom is the number of protons plus the number of neutrons. The atomic number of an atom in an un-ionized state is the number of protons or the number of electrons that are equal to each other.
The chemical properties of an atom are determined by its atomic number. Therefore, the number of neutrons in an atom can vary without changing its chemical properties. Oxygen, for example, has 8 protons, but different oxygen atoms can have either 8, 9, or 10 neutrons with corresponding mass numbers of 16, 17, and 18. These different nuclides of oxygen are its isotopes. Atoms can be split or caused to split artificially by bombardment with neutrons. However, some isotopic forms of certain elements undergo spontaneous disintegration because the nuclear forces cannot bind the excess numbers of neutrons with the protons that are inside the nucleus. The net result will be the breakdown of the nuclei into different particles. This is radioactivity. Some isotopes do not undergo spontaneous disintegration. They are called stable isotopes, and they are of little use in geochronology.
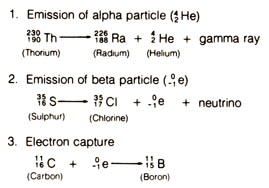
The nuclei of radioactive atoms can break down in several ways. The first is by losing a fragment containing two neutrons and two protons. This corresponds to the nucleus of a helium atom having a mass number of four. The process is called the emission of an alpha particle. The loss of an alpha particle reduces the number of neutrons and protons in the parent atom each by two thus changing it to a new element with a different set of chemical properties. The emission of alpha particles also brings the parent [61] nucleus to an excited state that is not stable. The nucleus will soon return to its stable ground state by losing the energy it has in the excited state through the emission of gamma rays.
A second way of radioactive disintegration is by the ejection of an electron or a beta particle. It is derived from the neutron in the nucleus. The loss of the electron causes the neutron to acquire a positive charge, and it turns into a proton. Therefore, after a beta emission the atomic number of the atom increases by one while the loss of the negligible mass of the beta particle does not change the mass number. A small particle called a neutrino is also given off accompanying a beta emission while the parent atom is changed to a new element.
The third type of radioactive decay is called electron capture. In this type the nucleus of an atom captures an electron from its innermost orbital shell accompanied by the emission of a neutrino and a gamma ray. This results in the loss of one proton whose positive charge has been neutralized by the negative charge of the electron while maintaining the same mass number. The atomic number is decreased by one and a new element is formed. Figure 2.1 summarizes the three types of radioactive decay.

Some radioactive isotopes may decay in two or more ways, and the choice of which alternative paths to take is purely random.Figure 2.1. Three types of radioactive decay. Superscripts are mass numbers. Subscripts are atomic numbers.
(2) Quantitation of Radioactive Decay and Age Determination. Radioactivity can be treated as the probability that a given nuclide will spontaneously disintegrate into another nuclide. If the probability is very small, the nuclide is a stable isotope. If it is large, then the nuclide is highly radioactive. If an individual radioactive nuclide is considered, it is impossible to predict exactly when it will decay. One can only estimate the probability that certain atoms will disintegrate within a given span of time. However, by taking large numbers of radioactive atoms into account and measuring the average rate of decay, a prediction of the proportion [62] that will have disintegrated within a certain time can be obtained.
The more radioactive nuclides present in the beginning, the higher the probability that radioactive disintegration will occur within a given span of time. Therefore, the number of atoms that actually undergo decay will be directly proportional to the number of parent atoms present originally. Since disintegration is a continuous process, the number of parent atoms will continually decrease while the number of daughter atoms will continually increase, provided that the daughter atoms are stable nuclides. A decay constant can be used to express the proportionality between the number of atoms decaying per unit time to the number of parent atoms. Each radioactive nuclide is characterized by a specific decay constant that is not affected by known physical or chemical processes. It is reasonable to assume, then, that the decay constants of radioactive nuclides in rocks used in geochronology also were unchanged during the geological time.
Since the quantitation of radioactivity is a statistical treatment, it is best to use a large sample and a long time span so that the detection of radioactivity will result in less fluctuation and a more accurate average decay rate. Because the atoms of a given radioactive nuclide decay at a certain average rate, it will require a definite amount of time for half of the initial atoms to undergo disintegration. This time is said to be the half-life of the radioactive nuclide. In other words, at the end of a period equal to one half-life, only half of the original radioactive atoms are left, and at the end of two half-lives, one quarter, and so on. The relationship between the decay constant, the original number of atoms present, and the elapsed time can be shown in the following equation:
Where
The negative sign indicates that the number of radioactive atoms decreases with time. By rearranging and integrating equation (1), the following equation is obtained.t = elapsed time= decay constant
N = number of atoms present
dn = change in # of radioactive atoms
Where
[63]No = number of atoms present at time = 0
e = base of natural logarithms
N = number of atoms present now.
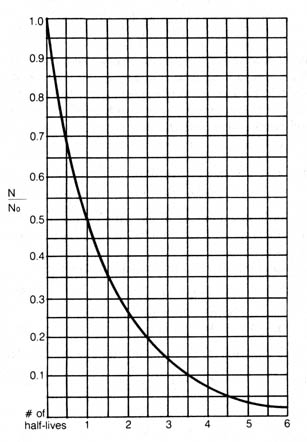
Figure 2.2. Graph illustrating law of radioactivity with proportion of radioactive parent remaining (N/No) drawn on line or scale.If we assume that the parent radioactive nuclides directly disintegrate into a single stable isotope or into a series of intermediate radioactive daughter atoms that eventually decay into a single stable isotope at a rate much faster than the rate of the decay of the parent atoms, we may adopt equation (2) to express relationship between the number of parent atoms existing at the beginning and at present. This equation then can be written: [64]
Where
Pi = the number of atoms of parent initially existingSince we have assumed the parent decays to daughter directly or indirectly but through a brief transient stage, the difference between the number of daughter atoms existing initially and the number of daughter atoms existing at present must be equal to the difference of Pp and Pi. This relationship can be expressed as:
Pp = the number of atoms of parent existing at present
t = elapsed time separating Pi and Pp
e = base of natural logarithms= decay constant.
Where
Di = number of atoms of daughter existing initiallyIn other words, the number of daughter atoms is constantly growing larger at the expense of the parent atoms whose number is constantly growing smaller.
Dp = number of atoms of daughter existing at present
By substituting equation (3) into equation (4) we get:
that can be rewritten as:
Rearranging equation (6) to solve for t, we get the following equation:
Equation (10) represents an adaptation of the fundamental equation (2) [65] for calculating age. Since one can determine the amount of daughter (Dp) and parent (Pp) existing at present by analysis and since the decay constant (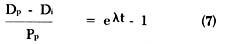

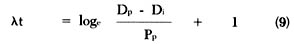
1. Geikie, A. The founders of geology. London:
Macmillan; 1905:50-51.
2. Geikie, A. The founders of geology. 79.
3. Lyell, C. Principles of geology. 9th ed.
New York: D. Appleton; 1853: 29.
4. Geikie, A. The founders of geology. 299.
5. Lyell, C. Principles of geology. 52.
6. Moore, J. R. J. Am. Sci. Affil. 22:18-23;
1970.
7. Velikovsky, I. Worlds in collision. New
York: Delta; 1950.
8. Steinhauser, L. J. Am. Sci. Affil. 25:129-33;
1973.
9. Yamauchi, E. M. J. Am. Sci. Affil. 25:134-39;
1973.