


2.1 Antiquity of the Earth (continued)
(3) Conventional Techniques of Radiometric Dating. There are
seven radiometric techniques used to date rocks. The carbon-14 (C-14) method
is used to date specimens that are 40,000 years old or younger. Methods
used to date rocks one million years of age and older include: uranium-238/lead-206,
uranium-235/lead-207, thorium-232/lead-208, lead-207/lead-206, potassium-40/argon-40,
and rubidium-87/strontium-87. Tables 2.1, 2.2, and 2.3 represent the sequences
of events and half-lives of the uranium-238/lead-206, uranium-235/lead-207,
and thorium-232/lead-208 series with respective half-lives of 4.51 x 109,
7.1 x 108, and 1.39 x 1010 [67] years. Figure 2.3
depicts the decay curves of each of these three series.
Table 2.1. The 238U (Uranium) series. Decay
constant of 238U is 1.54 x 10-10 per year.*
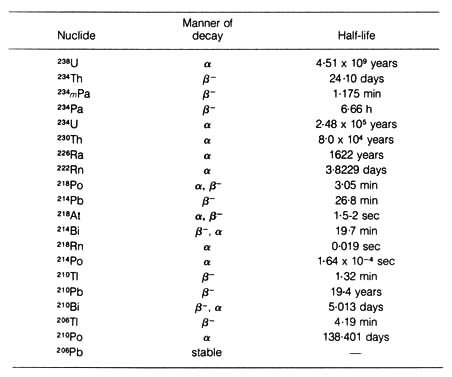
*NOTE: Adapted, with permission, from Hamilton, E. I.
Applied
geochronology.
London: Academic Press Inc.; 1965. © 1965 E. I.
Hamilton.
Table 2.1 400dpi 120k
[65]Table 2.2. The 235U (actinium) series.
Decay constant of
235U is 9.71 x 10-10 per year.*
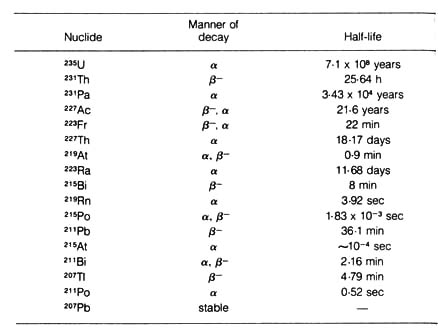
*NOTE: Adapted, with permission, from Hamilton, E. I.
Applied
geochronology.
London Academic Press Inc.; 1965. © 1965 E. I. Hamilton.
Table 2.2 400dpi 100k
Table 2.3. The 232Th (thorium) series. Decay
constant of
212Th is 4.99 x 10-10 per year.*

*NOTE: Adapted, with permission, from Hamilton, E. I.
Applied
geochronology.
Academic Press Inc.; 1965. © 1965 E. I. Hamilton.
Table 2.3 400dpi 80k
(a) Uranium-lead, Thorium-lead, Lead-lead Methods. The uranium-lead
and thorium-lead methods are based on the decay of uranium-238, uranium-235,
and thorium-232 into lead-206, lead-207, and lead-208 respectively, all
of which are stable isotopes. The decay of intermediate radioactive atoms
in all three series is much faster than the decay of the parent atoms.
Therefore, all the intermediate atoms are disregarded in the age calculation.
A fourth naturally occurring isotope of lead, lead-204 (common lead) is
not produced by radioactive decay, and it extremely stable. Uranium-238,
uranium-235, and thorium-232 frequently occur together, allowing at least
three independent age determinations from the same rock.
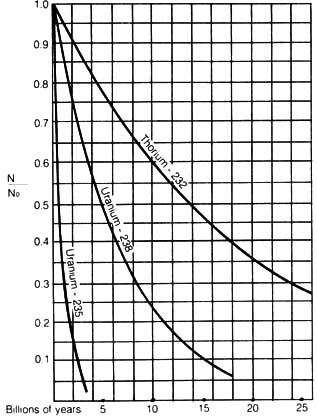
Figure 2.3. Decay curves for uranium - 235, uranium -
238, thorium - 232.

[68] Figure 2.4. Graphs showing changes in isotope
ratios in common lead in the earth during the past 4.5 billion years. Curves
are based on mass spectrometric analysis of many samples. Individual data
points are not shown. Reproduced, by permission, from Harbaugh, J. W. Stratigraphy
and the geologic time scale. 2nd ed. Dubuque, IA: Wm, C. Brown Co.
Publishers; 1974: 168. © Wm. C. Brown Co.
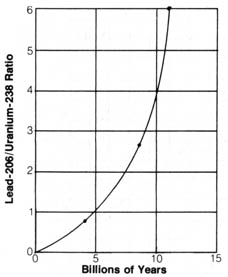
Figure 2.5. Graph showing ratio of lead-206/uranium-238 with
respect to time. Reproduced by permission, from Harbaugh, J. W. Stratigraphy
and the geologic time scale. 2nd ed. Dubuque, IA: Wm. C. Brown Co.
Publishers; 1974: 168. © 1974 Wm. C. Brown Co
The lead present with each of the above samples may not have been derived
from radioactive parents. The lead (common lead), assumed to have been
present when the mineral was formed (Di in equation [10]), must
be taken into consideration when age determination is to be carried out.
This can be done by correcting for the amount of common lead in the sample.
Geochronologists assume that when the earth was first [69] formed, the
proportion of the three lead isotopes (Pb 206, 207, 208) was fixed with
respect to common lead-204 (25).
In making age determinations it is necessary to subtract the common
lead from the radiogenic lead. Two things have to be kept in mind in doing
this: the approximate age of the mineral and the proportion of lead-204.
By referring to Figure 2.4, one can deduce the amount of lead 206, 207,
or 208 that is associated with the common lead. Then by subtracting these
quantities from the age equation (10), a more precise age can be determined.
Figure 2.5 represents the age determination by uranium-238/lead-206 after
corrections have been made.
In addition to the uranium-thorium-lead ratios, the ratio of lead-207
to lead-206 is useful in age determination. Since the half-life of uranium-235
(0.7 billion years) is much less than that of uranium-238 (4.5 billion
years), the amount of lead-207 produced by decay of uranium-235 increases
much more rapidly than that of lead-206 produced from uranium-238. Consequently,
the ratio of lead-207/lead-206 is a good indication of the age of rock
and is useful for dating specimens half a billion years old or older. Moreover,
the lead-207/lead-206 ratio is less likely to be affected by partial loss
of lead due to erosion, for both lead-207 and lead-206 have essentially
the same chemical properties; therefore, any loss of lead will probably
be the same. A graph of lead-207/lead-206 ratio versus time is shown in
Figure 2.6
(b) Potassium-argon Method. The potassium-argon method of age
dating has certain advantages over the uranium-thorium-lead methods because
potassium is more widely found among rocks in different areas. The element
potassium consists of three isotopes, namely, potassium-39, potassium-40,
and potassium-41, and only potassium-40 is radioactive. Potassium-40 decays
via two alternate routes, but 88% of the time it decays into calcium-40
by emitting a beta particle while 12% of the time it decays by electron
capture yielding argon-40. The half-life of either of these decay routes
is 1.3 x 109 years. Therefore, age determination can be carried
out either by potassium-40/calcium-40 ratio or by potassium-40/argon-40
ratio. But since nonradiogenic calcium-40 is ubiquitous, it tends to complicate
the dating procedure. Therefore, the potassium-40/argon-40 ratio is used
exclusively for dating purposes.
Argon-40 is a gas and is assumed to be absent from rocks during their
formation because of their high heat. Extreme care is taken by geochronologists
to insure the preservation of this gas in the rock. In order to insure
against loss of argon gas, a new technique involving the conversion of
potassium-39 to argon-39 is used. Neutron activation is followed by heating
the sample in stages to release argon-39 together with the argon-40. In
[70] a sample that has preserved the argon gas generated both radiogenically
and artificially, the two isotopes are expected to reside in fixed proportion
in equivalent crystal sites since they have both been produced from potassium.
Therefore, the ratio of argon-39/argon-40 can be related to potassium-40/argon-40
in a simple way (26, 27). Care is
also taken to detect the contamination of argon-40 by the argon in the
air. The argon-36 isotope present in the air is used for the detection
and subtraction of the amount of argon-40 absorbed by the sample from the
air. However, since the leakage of argon-40 sometimes poses a problem in
rocks that are known not to behave as a closed system, potassium-40/argon-40
dating is usually taken as the minimum age limit.

Figure 2.6. Graph of radiogenic lead-207/lead-206 ratio
versus time. Reproduced, by permission, from Harbaugh, J. W. Stratigraphy
and the geologic time scale. 2nd ed. Dubuque, IA: Wm. C. Brown Co.
Publishers; 1974: 168. © Wm, C. Brown Co.
(c) Rubidium-strontium. The rubidium-strontium method is
based on the decay of rubidium-87 to strontium-87 by the emission of a
beta particle with a half-life of 4.7 x 1010 years. Rubidium-87
is much more abundant than potassium and tends to occur in minerals that
are rich in potassium. Therefore, two independent dating methods can be
applied to the same sample containing potassium and rubidium.
(d) Carbon-14. The carbon-14 decay clock is widely used for
more recent samples (less than 40,000 years old). It is produced when a
nitrogen-14 atom absorbs a neutron in its nucleus and in turn emits a proton.
Carbon-14 decays by emitting a beta particle with a half-life of 5,730
years, reverting back to nitrogen-14. Carbon-14 is produced in the atmosphere
above 3,000 feet where nitrogen-14 is subjected to bombardment by neutrons
produced by cosmic rays, consisting of a large number of high-speed protons
that collide with the atmospheric gases. Newly created carbon-14 atoms
continually enter the world, and some of them [71] change into radioactive
carbon dioxide. Some of the radioactive carbon dioxide is taken up by photosynthetic
plants and enters the food chain of the living world. Thus, carbon-14 is
present in all living materials and forms a major internal radioactive
source inside the bodies of the organisms.
The carbon-14 dating method is based on the removal of the material
containing carbon-14 from the world exchange reservoir of carbon. As soon
as an organism dies and becomes fossilized, it will stop participating
in the food chain of the living world. Therefore, the amount of carbon-14
trapped in the dead organism is limited and will diminish with time. The
major assumption made in carbon-14 dating is that the carbon-14 in the
world remains at a steady level, i.e., the rate of formation of carbon-14
in the atmosphere by cosmic rays is equal to the rate of the decay of carbon-14
that already exists. This assumption was used to calculate the number of
disintegrations per minute (17.2 dpm) for the carbon-14 exchange reservoir
in the earth, and it agrees closely with the observed rate of 16.1 +
0.5 dpm (28). Therefore, the assumption is a reasonable
one. It is further assumed that the time required for exchange of carbon-14
in the organism with the environment is relatively short compared with
the subsequent time that is to be measured after the organism has died.
Thus, if a tree grew 200 years and yielded wood specimens that are 10,000
years old at present, the 200 years that the carbon-14 in the tree was
exchanged with the environment is relatively short compared with the present
age and will not enter into the calculation of age.
(4) Reliability of Age Determination and the Geological Column of
the Earth. The reliability of the age determination by radioactive
dating has been challenged (17, 23,
24).
Arguments used to refute the validity of the radioactive dating methods
include: the lack of assurance of the absence of environmental interchange
in the uranium/lead and thorium/lead dated rocks; the inaccurate estimate
of the contamination of the potassium-40/argon-40 clock by the argon-40
in the atmosphere; the unwarranted assumption of the time index in common
lead; and discrepancy in the carbon-14 exchange equilibrium hypothesis.
While many of these criticisms may be justified, the close agreement of
the results of the various radioactive methods when used to date rocks
from different parts of the world impress many because of their reproducibility.
Table 2.4 lists a comparison of the ages of the rocks taken from different
parts of the world as determined by five different radioactive dating methods.
The agreement, except in the first case, was well within Å l0%, a
degree of error commonly encountered in many scientific experiments. These
ages are termed concordant ages because of their close agreement.
There are well-documented cases of discordant ages, ages showing
large [72] discrepancy as determined by different methods, but they are
usually within a three- or fourfold difference (Table 2.5). Moreover the
discordant ages seem to follow a regular pattern, and in some cases they
can be explained by the contamination or loss of the parent intermediate,
final products in the sample, or by the continuous diffusion of lead from
the rock into the environment.
Table 2.4. Degree of concordance between age
estimates determined by different methods.*
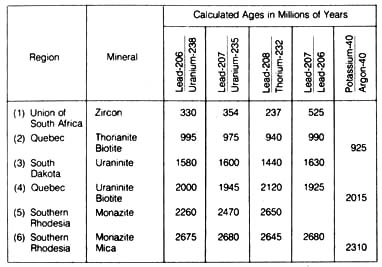
*NOTE: Reprinted, by permission, from Harbaugh,
J. W.
Stratigraphy and the geologic time scale. 2nd ed. Dubuque,
IA: Wm. C. Brown Company Publishers: 1974: 168. ©1974 Wm. C. Brown
Co.
Table 2.4 400dpi 132k
The quantitative analytical techniques utilized by geochronologists have
produced reliable information. The decay constants of most of the radioactive
elements used in the dating methods can be known to within at least 5%,
and in the case of uranium-238, uranium-235, and rubidium-87, the error
is probably less than 3%. The ratio of isotopes can also be accurately
measured to within 1-3% error. For example, five laboratories in the United
States analyzed a sample from the Department of Geophysics of Massachusetts
Institute of Technology. The reported values of argon-40 in the sample
were all within 1% of the mean. In a critical sample, several duplicate
analyses are routinely made, and intercalibrations on at least one sample
were made by all laboratories involved in the dating process to avoid systematic
errors (40). Therefore, gross experimental errors are
eliminated.
Table 2.5. Concordant and discordant ages based on
four dating methods. [73]
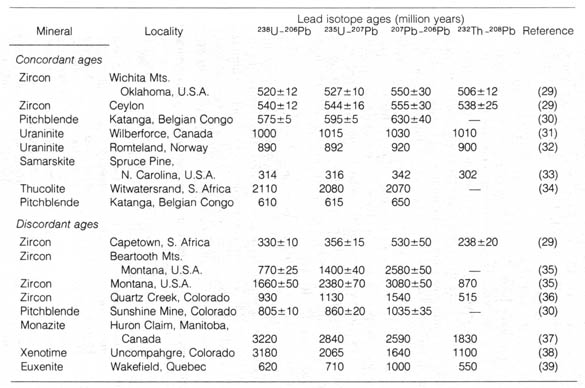
*NOTE: Adapted, with permission, from Hamilton,
E. I. Applied geochronology. London: Adcademic Press Inc.; 1965.
© 1965 E. I. Hamilton. Table 2.5 400dpi
275k
Recently six different methods of radioactive dating on the moon rocks,
[74] namely, uranium-238/lead-207; uranium-235/lead-208; thorium-232/ lead-208;
rubidium-87/strontium-87; argon-39/argon-40; and lead-207/lead-206, yielded
dates of 4.70; 4.67; 4.60; 3.4-4.5; 3.7; and 4.75 billion years, respectively
(41,
42,
43).
These are in close agreement with each other and with the age of the earth
that is estimated to be 4.5 billion years using the same methods.
It has been charged that since some of the noble gases found on the
lunar surface have been derived from the solar wind (a constant stream
of particles from the sun), the potassium-argon dating of moon rock is
unreliable (44). However, the potassium-argon dating
was done with the interior portions of the crystalline rocks thus avoiding
the outer portion of the rocks that may have been contaminated by the solar
wind. Moreover, analysis of the ratios of the noble gases in the lunar
surface showed that their concentrations were inversely proportional to
the particle size. However, these ratios were significantly different from
solar values. The ratios of the isotopes, on the other hand, were found
to be similar to those in meteorites. Corrections using the solar values
measured in the outer portions of crystalline rocks were routinely done
in the potassium-argon dating method, and these solar corrections were
found to be negligible in almost all cases (45, 46,
47).
The moon was believed to have been heated up "recently" to temperatures
between 1000°C and 1300°C. At such heat all the elements used in
radiometric dating would have been melted, thus rendering the dating methods
useless (44). This view apparently was not held in high
regard by the moon-rock scientists, for none of the articles in the "Moon"
issue of Science
(30 January 1970) referred to it. It was estimated,
however, that
Table 2.6. Comparison of ages obtained by radiocarbon
dating with material of known ages. L (50, 51);
B (52); C (53).*
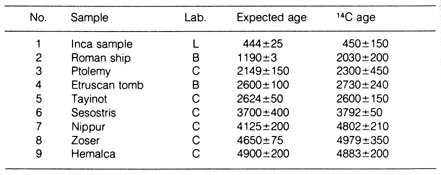 *NOTE: Reprinted, with permission, from Hamilton, E. I.
Applied
geochronology.
*NOTE: Reprinted, with permission, from Hamilton, E. I.
Applied
geochronology.
London: Academic Press Inc.; 1965. © 1965 E. I.
Hamilton.
[75] the moon was crystallized at a temperature range of 1140°C to
1170°C and that it had been reduced to a greater extent during crystallization
than had the earth (48). The assumptions in the radiometric
dating were apparently that the radiogenic elements were derived from components
that surfaced during the process of crystallization. Since the surface
of the moon is atmosphere-free (49), the erosion and
diffusion processes that are usually associated with the contamination
and loss of radioactive atoms in a sample rock are minimized. The remarkable
agreement of the age of moon rock with that of earth rock increased the
credence of the radioactive dating methods.
A comparison of the carbon-14 dated samples with known ages also indicated
that the method is reliable within the limits of experimental errors (Table
2.6). Recently a new carbon-14 dating method was introduced that utilizes
a cyclotron that increases the maximum determinable age to 100,000 years
while reducing the sample size required. Scientists hope that this will
increase the efficiency of the carbon-14 method (54).
A new method of dating human fossils too old for C-14 that depends on
the constant rate of the racemization of amino acids has also been developed.
This process changes an optically active compound that can rotate plane
polarized light into an optically inactive or racemic mixture. Since only
L-amino acids are found in living organisms, the D-amino acids detected
on human fossils can be attributed to the process of racemization. Of particular
interest in dating because of its long-time constant and ease of measurement
is the reaction involving L-isoleucine and D-allo-isoleucine. L-isoleucine
and D-allo-isoleucine are separable on the buffered columns of the automatic
amino acid analyzer.
Constant temperature is the critical requirement in dating by racemization
of amino acids. Using this method, fossils found in caves with relatively
constant environments can be dated with only Å5 to 10% uncertainty.
Samples within the C-14 range dated by this method have yielded concordant
ages with those using C-14 dating. The maximum age that can be obtained
by this method for caves with temperatures of 10°C and 20°C would
be approximately 1.3 x 106 and 1.9 x 105 years respectively.
This method has become quite useful for dating human fossils between 40,000
and 1 million years of age (55).
Each geological stratum in the earth's crust (geological column) has
been correlated with quantitative measurements of radiometric data, resulting
in the construction of a geologic time scale. Dated materials are used
as tiepoints between geologic strata in constructing the time scale. The
best candidates for tiepoints are layered volcanics, which consist of lava
flows and deposits of volcanic ash. They have the advantage of having [76]
Table 2.7. Radiometric dates that have been applied
to geologic time scale.*
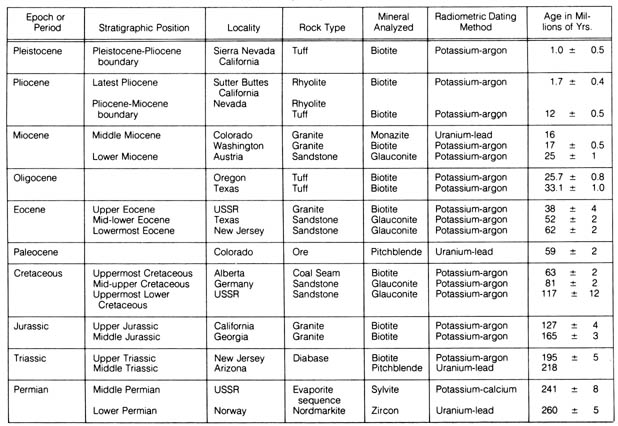
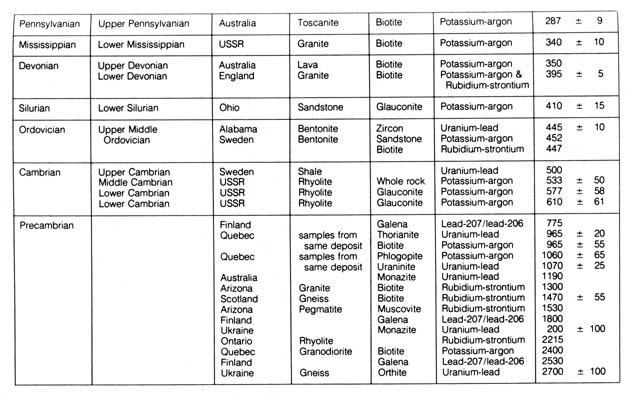 *NOTE: Reprinted, by permission, from Harbaugh, J. W.
Stratigraphy
and the geologic time scale. 2nd ed. Dubuque, IA: Wm. C. Brown Co.
Publishers; 1974. © 1974 Wm. C. Brown Co. Table
2.7a 400dpi 520k Table 2.7b 400dpi 404k
*NOTE: Reprinted, by permission, from Harbaugh, J. W.
Stratigraphy
and the geologic time scale. 2nd ed. Dubuque, IA: Wm. C. Brown Co.
Publishers; 1974. © 1974 Wm. C. Brown Co. Table
2.7a 400dpi 520k Table 2.7b 400dpi 404k
[78] been deposited quickly, and often they occur interstratified
within a sequence of fossiliferous sedimentary rocks, permitting an establishment
of their position in relation to the layers of rock.
Furthermore, layered volcanics frequently contain minerals used in several
radioactive dating techniques. Many volcanic rocks have yielded concordant
ages, lending credence to the geological time scale. Other rocks, such
as bracket intrusives and glauconites, are used occasionally as tiepoints.
However, they are problematic either because they span too many stratigraphic
layers, or they are continually being formed after the sedimentation of
the layer has been completed. Despite these shortcomings, the geological
time scale has as much validity as is allowed by the current stratus of
geochronological science. Table 2.7 summarizes the geological column as
correlated by representative samples to the absolute radiometric age.



References 2.1
25. Russell, R. D.; Farquhar, R. M.
Lead
isotopes and geology. New York: Inter Science; 1960.
26. Turner, G.
Science. 167:466-68;
1970.
27. Turner, G.
Meteorite research.
Dordrecht, Holland: Reidell; 1969.
28. Hamilton, E. I.
Applied geochronology.
41.
29. Tilton, G. R.; Davis; G. L.; Wetherill;
G. W.; Aldrich, L. T.
Trans. Am. Geophy. Un. 38:360; 1957.
30. Eckelmann, W. R.; Kulp, J. L.
Bull.
Geol. Soc. Am. 67:35; 1956.
31. Nier, A. O.
J. Appl. Phys. 12:342;
1941.
32. Kulp J. L.; Eckelmann, W. R.
Am.
Min. 42:154; 1957.
33. Eckelmann, W. R.; Kulp, J. L.
Bull.
Geol. Soc. Am. 68:1117; 1957.
34. Louw, J. D.
Nature. 175:349;
1955.
35. Catanzaro, E. J.; Kulp, J. L.
Geochim.
Cosmachim. Acta. 28:87; 1964.
36.
Carnegie Report. 1954-55.
37. Nier, A. O.
Physiol. Rev. 55:150;
1939.
38. Tilton, G. E.
Trans. Am. Geophys.
Un. 32 (2):224; 1956.
39. Robinson, S. L.; Loveridge, W. D.;
Rimsaite, J.; van Petegehm,
J. Canad. Min. 17 (3):533; 1963.
40. Kulp, J. L.
Science. 113:1105;
1961.
41. Tatsumoto, M.; Rosnolt, J. N.
Science.
167:461-63;
1970.
42. Turner, G.
Science. 167:466-68;
1970.
43. Gopalan, K. et al.
Science.
167:471-73; 1970.
44. Coppedge, J. F.
Evolution: possible
or impossible? Grand Rapids, MI: Zondervan; 1973: 250.
45. Heymann, D. et al.
Science.
167:555-58; 1970.
46. Eberhardt, P. et al.
Science. 167:558-60;
1970.
47. Funkhouser, J. G. et al.
Science.
167:561-63; 1970.
[80]
48. Anderson, A. T. et al.
Science.
167:587-89; 1970.
49. Lunar sample analysis planning team.
Summary of Apollo 11 lunar science conference.
Science. 167:450-51;
1970.
50. Kulp, J. L.; Feely, W. H.; Tryon, L.
E.
Science. 114:565; 1951.
51. Kulp, J. L.; Volchok, H. L.; Holland,
H. D.
Trans. Am. Geophy. Un. 33:101; 1952.
52. Ballaria, C.
Science. 121:409;
1955.
53. Arnold, J. R.; Libby, W. F.
Science.
110:678; 1949. 54. Muller, R. A.
Science. 196:489; 1977.
54. Muller, R. A.
Science. 196:489;
1977.
55. Bishop, W. W.; Miller, J. A., editors.
Recent advances in isotope and other dating methods applicable to the origin
of man. Edinburgh: Scottish Academic Press; 177-85; 1972.













